Abstract
Aims
Root system architecture traits (RSAT) are crucial for crop productivity, especially under drought and low soil fertility. The “shovelomics” method of field excavation of mature root crowns followed by manual phenotyping enables a relatively high throughput as needed for breeding and quantitative genetics. We aimed to develop a new sampling protocol in combination with digital imaging and new software.
Methods
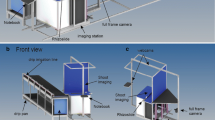
Sampled rootstocks were split lengthwise, photographed under controlled illumination in an imaging tent and analysed using Root Estimator for Shovelomics Traits (REST). A set of 33 diverse maize hybrids, grown at 46 and 192 kg N ha−1, was used to evaluate the method and software.
Results
Splitting of the crowns enhanced soil removal and enabled access to occluded traits: REST-derived median gap size correlated negatively (r = −0.62) with lateral root density based on counting. The manually measured root angle correlated with the image-derived root angle (r = 0.89) and the horizontal extension of the root system (r = 0.91). The heritabilities of RSAT ranged from 0.45 to 0.81, comparable to heritabilities of plant height and leaf biomass.
Conclusion
The combination of the novel crown splitting method, combined with imaging under controlled illumination followed by automatic analysis with REST, allowed for higher throughput while maintaining precision. The REST Software is available as supplement.









Similar content being viewed by others
References
Abendroth LJ, Elmore RW, Boyer MJ, Marlay SK (2011) Corn growth and development. PMR 1009. Iowa State Univ. Extension, Ames
Araki H, Hirayama M, Hirasawa H, Iijima M (2000) Which roots penetrate the deepest in rice and maize root systems. Plant Prot Sci 3:281–288
Bucksch A, Burridge J, York LM et al (2014) Image-based high-throughput field phenotyping of crop roots. Plant Physiol 166:470–486. doi:10.1104/pp. 114.243519
Burton AL, Brown KM, Lynch JP (2013) Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Sci 53:1–15
Cai H, Chen F, Mi G et al (2012) Mapping QTLs for root system architecture of maize (Zea mays L.) in the field at different developmental stages. Theor Appl Genet 125:1313–24. doi:10.1007/s00122-012-1915-6
Campos H, Cooper M, Habben JE et al (2004) Improving drought tolerance in maize: a view from industry. Fields Crop Res 90:19–34
Chun L, Mi G, Li J et al (2005) Genetic analysis of maize root characteristics in response to low nitrogen stress. Plant Soil 276:369–382
Coque M, Martin A, Veyrieras JB et al (2008) Genetic variation for N-remobilization and postsilking N-uptake in a set of maize recombinant inbred lines. 3. QTL detection and coincidences. Theor Appl Genet 117:729–747
Falconer DS, Mackay TF (1996) Introduction to quantitative genetics, 4th edn. Longman, Harlow
Gallais A, Coque M (2005) Genetic variation and selection for nitrogen use efficiency in maize: a synthesis. Maydica 50:531–547
Gallais A, Hirel B (2004) An approach to the genetics of nitrogen use efficiency in maize. J Exp Bot 55:295–306
Gaudin ACM, McClymont SA, Holmes BM et al (2011) Novel temporal, fine-scale and growth variation phenotypes in roots of adult-stage maize (Zea mays L.) in response to low nitrogen stress. Plant Cell Environ 34:2122–2137
Giuliani S, Sanguineti MC, Tuberosa R et al (2005) Root-ABA1, a major constitutive QTL, affects maize root architecture and leaf ABA concentration at different water regimes. J Exp Bot 56:3061–3070
Grieder C, Trachsel S, Hund A (2014) Early vertical distribution of roots and its association with drought tolerance in tropical maize. Plant Soil 377:295–308. doi:10.1007/s11104-013-1997-1
Grift TE, Novais J, Bohn M (2011) High-throughput phenotyping technology for maize roots. Biosyst Eng 110:40–48
Guilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ASReml user guide release 3.0. VSN Int. Ltd, Hemel Hempstead, HP1 1ES, UK
Hammer G, Dong Z, McLean G et al (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Sci 49:299–312
Hochholdinger F (2009) The maize root system: morphology, anatomy, and genetics. In: Hake SC, Bennetzen JL (eds) Handb. maize Its Biol. Springer New York, New York, pp 145–160
Hochholdinger F, Tuberosa R (2009) Genetic and genomic dissection of maize root development and architecture. Curr Opin Plant Biol 12:172–177
Hund A (2010) Genetic variation in the gravitropic response of maize roots to low temperatures. Plant Roots 4:22–30. doi:10.3117/plantroot.4.22
Hund A, Fracheboud Y, Soldati A et al (2004) QTL controlling root and shoot traits of maize seedlings under cold stress. Theor Appl Genet 109:618–29
Hund A, Reimer R, Messmer R (2011) A consensus map of QTLs controlling the root length of maize. Plant Soil 344:143–158
Iyer-Pascuzzi AS, Symonova O, Mileyko Y et al (2010) Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiol 152:1148–1157
Kahn BA, Stoffella JP (1991) Nodule distribution among root morphological components of field-grown cowpeas. J Am Soc Hortic Sci 116:655–658
Ku LX, Sun ZH, Wang CL et al (2012) QTL mapping and epistasis analysis of brace root traits in maize. Mol Breed 30:697–708
Kumar B, Abdel Ghani AH, Reyes-Matamoros J et al (2012) Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines. Plant Breed 131:465–478
Kutscherea L, Lichtenegger E (1960) Wurzelatlas mitteleuropäischer Ackerunkräuter und Kulturpflanzen. DLG-Verlag, Frankfurt am Main
Liedgens M, Soldati A, Stamp P, Richner W (2000) Root development of maize (Zea mays L.) as observed with minirhizotrons in lysimeters. Crop Sci 40:1665–1672
Liu J, Li J, Chen F (2008) Mapping QTLs for root traits under different nitrate levels at the seedling stage in maize (Zea mays L.). Plant Soil 305:253–265
Lynch JP (1995) Root architecture and plant productivity. Plant Physiol 109:7–13
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot. doi:10.1093/aob/mcs293
Maddonni GA, Otegui E, Andrieu B et al (2002) Maize leaves turn away from neighbors. Plant Physiol 130:1181–1189. doi:10.1104/pp. 009738.nated
Manavalan LP, Musket T, Nguyen HT (2012) Natural genetic variation for root traits among diversity lines of maize (Zea Mays L.). Maydica 56
Moisy F (2006) “Boxcount” (Matlab Central, 2006). http://www.mathworks.com/matlabcentral/fileexchange/13063-boxcount. Accessed 30 May 2013
Nielsen KL, Lynch JP, Weiss HN (1997) Fractal geometry of bean root systems: correlations between spatial and fractal dimension. Am J Bot 84:26–33
Nielsen KL, Miller CR, Beck D, Lynch JP (1999) Fractal geometry of root systems: field observations of contrasting genotypes of common bean (Phaseolus vulgaris L.) grown under different phosphorus regimes. Plant Soil 206:181–190
Otsu N (1979) A threshold selection method from gray-level histogrmas. IEEE Trans Syst Man Cybern 9:62–6
Passioura JB (2012) Phenotyping for drought tolerance in grain crops: when is it useful to breeders? Funct Plant Biol 39:851–859
Piepho H-P, Möhring J (2007) Computing heritability and selection response from unbalanced plant breeding trials. Genetics 177:1881–1888
R Core Team (2013) R: a language and environment for statistical computing
Ruta N, Liedgens M, Fracheboud Y (2010) QTLs for the elongation of axile and lateral roots of maize in response to low water potential. Theor Appl Genet 120:621–631
Saengwilai P, Tian X, Lynch JP (2014) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166:581–9. doi:10.1104/pp. 113.232603
Singh V, Oosterom EJ, Jordan DR et al (2010) Morphological and architectural development of root systems in sorghum and maize. Plant Soil 333:287–299
Stoffella PJ, Sandsted RF, Zobel RW, Hymes WL (1979) Root characteristics of black beans. II. Morphological differences among genotypes. Crop Sci 19:826–830
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341:75–87
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2013) Maize root growth angles become steeper under low N conditions. Fields Crop Res 140:18–31
Vamerali T, Saccomani M, Bona S et al (2003) A comparison of root characteristics in relation to nutrient and water stress in two maize hybrids. Plant Soil 255:157–167
Vargas M, Combs E, Alvarado G et al (2013) META: a suite of SAS programs to analyze multienvironment breeding trials. Agron J 105:11–19
Walk TC, Van Erp E, Lynch JP (2004) Modelling applicability of fractal analysis to efficiency of soil exploration by roots. Ann Bot 94:119–28. doi:10.1093/aob/mch116
Weaver JE (1925) Investigations on the root habits of plants. Am J Bot 12:502–509
Wiesler F, Horst WJ (1994) Root growth and nitrate utilization of maize cultivars under field conditions. Plant Soil 163:267–277
Worku M, Bänziger M, Friesen D, Diallo AO, Horst WJ (2012) Nitrogen efficiency as related to dry matter partitioning and root system size in tropical mid-altitude maize hybrids under different levels of nitrogen stress. Fields Crop Res 130:57–67
Wu L, McGechan MB, Watson CA, Baddeley JA (2005) Develping existing plant root system architecture models to meet future agricultural challenges. Adv Agron 85:181–219
York LM, Nord EA, Lynch JP (2013) Integration of root phenes for soil resource acquisition. Front Plant Sci 4:1–15. doi:10.3389/fpls.2013.00355
Yu G-R, Zhuang J, Nakayama K, Jin Y (2007) Root water uptake and profile soil water as affected by vertical root distribution. Plant Ecol 189:15–30
Zhong D, Novais J, Grift TE et al (2009) Maize root complexity analysis using a support vector machine method. Comput Electron Agric 69:46–50
Zhu J, Kaeppler SM, Lynch JP (2005a) Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor Appl Genet 111:688–95. doi:10.1007/s00122-005-2051-3
Zhu J, Kaeppler SM, Lynch JP (2005b) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays). Funct Plant Biol 32:749. doi:10.1071/FP05005
Zhu J, Ingram PA, Benfey PN, Elich T (2011) From lab to field, new approaches to phenotyping root system architecture. Curr Opin Plant Biol 14:310–317
Zobel RW (2011) A developmental genetic basis for defining root classes. Crop Sci 51:1410. doi:10.2135/cropsci2010.11.0652
Zobel RW, Waisel Y (2010) A plant root system architectural taxonomy: a framework for root nomenclature. Plant Biosyst 144:507–512. doi:10.1080/11263501003764483
Acknowledgments
The authors would like to thank the anonymous reviewers for their helpful suggestions and Achim Walter for his support. Thanks for assistance in the field go to Johan Prinsloo and the farmworkers in South Africa. Thanks to Claude Welcker for his assistance in assembling the EURoot maize panel and to Delley Seeds and Plants Ltd. for hybrid production. We kindly thank the donors of the genetic material: Department of Agroenvironmental Science and Technologies (DiSTA), University of Bologna, Italy (RootABA lines); Misión Biológica de Galicia (CSIC), Spain (EP52); Estación Experimental de Aula Dei (CSIC), Spain (EZ47, EZ11A, EZ37); Centro Investigaciones Agrarias de Mabegondo (CIAM), Spain (EC169); Misión Biológica de Galicia (CSIC), Spain, (EP52); University of Hohenheim, Versuchsstation für Pflanzenzüchtung, Germany (UH007, UH250); and INRA CNRS UPS AgroParisTech, France (supply of the remaining, public lines). We thank the Forschungszentrum Jülich GmbH, Germany for the MatLib package and Oliver Dressler for the CAD illustrations. Support for field research in South Africa was provided to Jonathan Lynch by the Howard G. Buffett Foundation. This research received funding from the European Community Seventh Framework Programme FP7-KBBE-2011-5 under grant agreement no.289300 and the Walter Hochstrasser-Stiftung.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter J. Gregory.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Image processing with RootEstimatorForShovelomicsTraits from binary images: Determination of fractal dimensions by stepwise reduction of the grid fineness, here exemplary: a) 512*512 mashes, b) 256*256 mashes, c) 128*128 mashes and d) 64*64 mashes. (TIFF 248 kb)
Figure S2
Scatter plot and Pearson correlation coefficients between genotype means of root top angle (AngRt) and a) root angle of the youngest whorl (AngNo-0), b) the second youngset whorl (AngNo-1) and c) the third youngest whorl (AngNo-2); (**) denotes significant correlations on p-level 0.01, (n.s.) denotes non-siginificant correlations. (TIFF 86 kb)
Figure S3
Scatter plots and Pearson correlation coefficients between genotype means of the area of the convex hull (AcH) and a) nodal root number at the youngest whorl (#NoNo-0) and b) the projected total structure length; (**), (°) denote significant correlations on p-level 0.01 and 0.1 respectively. (TIFF 68 kb)
Figure S4
Scatter plots and Pearson correlation coefficients between genotype means of the leaf fresh weight (FWLf) and a) nodal root number at the youngest whorl (#NoNo-0), b) the area of the convex hull (AcH) and c) the fractal dimension (FD); (**), (*) denote significant correlations on p-level 0.01 and 0.05 respectively, (n.s.) denotes non-siginificant correlations. (TIFF 80 kb)
Figure S5
Scatter plots and Pearson correlation coefficients between genotype means of the a) branching density of lateral roots at 3rd youngest whorl (BDNo-2) and the median gap size and b) the filling factor (Ff) in the convex hull and the median gap size; (*) denote significant correlations on p-level 0.05. (TIFF 72 kb)
Figure S6
Mean root angles of the youngest (AngNo-0), second youngest (AngNo-1) and third youngest (AngNo-2) nodal root whorl of each genotype under high (HN; open circles) or low (LN; cross) nitrogen. (TIFF 136 kb)
ESM 7
(ZIP 26370 kb)
Rights and permissions
About this article
Cite this article
Colombi, T., Kirchgessner, N., Le Marié, C.A. et al. Next generation shovelomics: set up a tent and REST. Plant Soil 388, 1–20 (2015). https://doi.org/10.1007/s11104-015-2379-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2379-7