Abstract
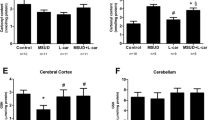
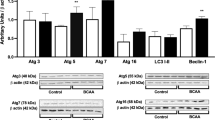
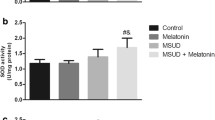
In the present work we investigated the in vitro effect of the branched-chain amino acids (BCAA) accumulating in maple syrup urine disease (MSUD) on some parameters of energy metabolism in cerebral cortex of rats. 14CO2 production from [1-14C]acetate, [1-5-14C]citrate and [U-14C]glucose, as well as glucose uptake by the brain were evaluated by incubating cortical prisms from 30-day-old rats in the absence (controls) or presence of leucine (Leu), valine (Val) or isoleucine (Ile). All amino acids significantly reduced 14CO2 production by around 20–55%, in contrast to glucose utilization, which was significantly increased by up to 90%. Furthermore, Leu significantly inhibited the activity of the respiratory chain complex IV, whereas Val and Ile markedly inhibited complexes II–III, III and IV by up to 40%. We also observed that trolox (α-tocopherol) and creatine totally prevented the inhibitory effects provoked by the BCAA on the respiratory chain complex activities, suggesting that free radicals were involved in these effects. The results indicate that the major metabolites accumulating in MSUD disturb brain aerobic metabolism by compromising the citric acid cycle and the electron flow through the respiratory chain. We presume that these findings may be of relevance to the understanding of the pathophysiology of the neurological dysfunction of MSUD patients.






Similar content being viewed by others
References
Chuang DT, Shih VE (2001) Maple Syrup Urine Disease (Branched-chain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WL, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 1971–2005
Yeman SJ (1986) The mammalian 2-oxoacid dehydrogenase: a complex family. Trends Biochem Sci. 11:293–296
Treacy E, Clow CL, Reade TR et al (1992) Maple syrup urine disease: interrelationship between branched chain amino-, oxo-, and hydroxyacids; implications for treatment; association with CNS dysmelination. J Inher Metab Dis 15:121–135
Nyhan WL (1984) Abnormalities in amino acid metabolism in clinical medicine. Appleton-Centurty-Crofts, Norwalk, pp 21–35
Scriver CR, Clow CL, George HG (1985) So-called thiamin-responsive maple syrup urine disease: 15-year follow-up of the original patient. J Pediatr 107:763–765
Snyderman SE, Norton PM, Roitman E (1964) Maple syrup urine disease with particular reference to diet therapy. Pediatrics 34:454–472
Efron ML (1965) Aminoaciduria. N Engl J Med 272:1058–1067
Danner DJ, Elsas II LJ (1989) Disorders of branched chain amino acid and keto acid metabolism. In: Scriver CR, Beaudet AL, Sly WL, Valle D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 671–692
Tribble D, Shapira R (1983) Myelin proteins: degradation in rat brain initiated by metabolites causative of maple syrup urine disease. Biochem Biophys Res Comm 114:440–446
Taketomi T, Kunishita T, Hara A et al (1983) Abnormal protein and lipid compositions of the cerebral myelin of a patient with maple syrup urine disease, Jpn J Exp Med 53:109–116
Tashian RE (1961) Inhibition of brain glutamic acid decarboxylase by phenylalanine, valine, and leucine derivatives: a suggestion concerning the etiology of the neurological defect in phenylketonuria and branched-chain ketonuria. Metabolism 10:393–402
Yuwiler A, Geller E (1965) Serotonin depletion by dietary leucine. Nature 208:83–84
Zielke HR, Huang Y, Tildon JT et al (1996) Elevation of amino acids in the interstitial space of the rat brain following infusion of large neutral amino and keto acids by microdialysis: leucine infusion. Dev Neurosci 18:420–425
Tavares RG, Santos CES, Tasca C et al (2000) Inhibition of glutamate uptake into synaptic vesicles of rat brain by the metabolites accumulating in maple syrup urine disease. J Neurol Sci 181:44–49
Araújo P, Wassermann GF, Tallini K et al (2001) Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int 38:529–537
Fontella FU, Gassen E, Pulrolnik V et al (2002) Stimulation of lipid peroxidation in vitro in rat brain by metabolites accumulating in maple syrup urine disease. Metab Brain Dis 17:47–54
Bridi R, Araldi J, Sgarbi MB (2003) Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int J Dev Neurosci 21:327–332
Bridi R, Fontella FU, Pulrolnik V (2006) A chemically-induced acute model of maple syrup urine disease in rats for neurochemical studies. J Neurosci Methods 155:224–230
Jouvet P, Rustin P, Taylor DL et al (2000) Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane despolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell 11:1919–1932
Howell RK, Lee M (1963) Influence of α-keto acids on the respiration of brain in vitro. Proc Soc Exp Biol Med 113:660–663
Halestrap AP, Brand MD, Denton RM (1974) Inhibition of mitochondrial pyruvate transport by phenylpyruvate and α-ketoisocaproate. Biochem Biophys Acta 367:102–108
Land JM, Mowbray J, Clark JB (1976) Control of pyruvate and β-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J Neurochem 26:823–830
Pilla C, Cardozo RF, Dutra-Filho CS (2003) Creatine kinase activity from rat brain is inhibited by branched-chain amino acids in vitro. Neurochem Res 28:675–679
Sgaravati AM, Rosa RB, Schuck PF et al (2003) Inhibition of brain energy metabolism by the α-keto acids accumulating in maple syrup urine disease. Biochem Biophys Acta 1639:232–238
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Dutra-Filho CS, Wajner M, Wannmacher CM et al (1995) 2-hydroxybutyrate and 4-hyddroxybutyrate inhibit CO2 formation from labeled substrates by rat cerebral cortex. Biochem Soc Trans 23:228S
Sorensen RG, Mahler HR (1982) Localization of endogenous ATPases at the nerve terminal. J Bioenerg Biomembr 14:527–547
Shepherd D, Garland PB (1973) Citrate synthase from rat liver. Methods Enzymol 13:11–16
Plaut GWE (1969), Isocitrate dehydrogenase from bovine heart. In: Lowenstein JM (ed) Methods in enzymology 13, Academic Press, New York, pp 34–42
Humphries KM, Szweda LI (1998) Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry 37:15835–15841
Fisher JC, Ruitenbeek W, Berden JÁ et al (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Birch-Machin MA, Briggs HL, Saborido AA et al (1994) An evaluation of the measurement of the activities of complexes I–IV in the respiratory chain of human skeletal muscle mitochondria. Biochem Med Metab Biol 51:35–42
Rustin P, Chretien D, Bourgeron T et al (1994) Biochemical and Molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
da Silva CG, Ribeiro CAJ, Leipnitz G et al (2002) Inhibition of cytochrome c oxidase activity in rat cerebral cortex and human skeletal muscle by D-2-hydroxyglutaric acid in vitro. Biochim Biophys Acta 1586:81–91
Trinder PA (1969) Determination of blood glucose using on oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161
Dutra JC, Wajner M, Wannmacher CF et al (1991) Effects of methylmalonate and propionate on glucose and ketone bodies uptake in vitro by brain of developing rats. Biochem Med Metab Res 45:56–64
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Loaiza A, Porras OH, Barros LF (2003) Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy, J Neurosci 23:7337–7342
Pellerin L, Magistretti PJ (2004) Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 10:53–62
Yudkoff M, Daikhin Y, Lin Z-P et al (1994) Interrelationships of leucine and glutamate metabolism in cultured astrocytes. J Neurochem 62:1192–1202
Hutson SM, Lieth E, LaNoue KF (2001) Function of leucine in excitatory neurotransmitter metabolism in the central nervous system. J Nutr 131:846S–850S
Chang TW, Goldberg AL (1978) Leucine inhibits oxidation of glucose and pyruvate in skeletal muscles during fasting. J Biol Chem 253:3696–3701
Tretter L, Szabados G, Ando A et al (1987) Effect of succinate on mitochondrial lipid peroxidation. II. The protective effect of succinate against functional and structural changes induced by lipid peroxidation. J Bioenerg Biomembr 19:31–44
Bindoli A (1988) Lipid peroxidation in mitochondria. Free Rad Biol Med 5:247–261
Cardoso SM, Pereira C, Oliveira R (1999) Mitochondrial function is differentially affected upon oxidative stress. Free Radic Biol Med 26:3–13
Felber SR, Sperl W, Chemelli A, Murr C, Wendel U (1993) Maple syrup urine disease: metabolic decompensation monitored by proton magnetic resonance imaging and spectroscopy. Ann Neurol 33:396–401
Sener RN (2007) Maple syrup urine disease: diffusion MRI, and proton MR spectroscopy findings. Comput Med Imaging Graphics 31:106–110
Jan W, Zimmerman RA, Wang ZJ, Berry GT, Kaplan PB, Kaye EM (2003) MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology 45:393–399
Doi M, Ymaoka I, Nakayama M et al. (2005) Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J Nutr 135:2103–2108
Funchal C, Schuck PF, Santos AQ et al (2006) Creatine and antioxidant treatment prevent the inhibition of creatine kinase activity and the morphological alterations of C6 glioma cells induced by the branched-chain alpha-keto acids accumulating in maple syrup urine disease. Cell Mol Neurobiol 26:67–79
Jackson RH, Singer TP (1983) Inactivation of the 2-ketoglutarate and pyruvate dehydrogenase complexes of beef heart by branched chain keto acids, J Biol Chem 258:1857–1865
Gibson GE, Blass JP (1976) Inibition of acetylcholine synthesis and of carbohydrate utilization by maple syrup urine disease metabolites. J Neurochem 26:1073–1078
Walajtys-Rode E, Williamson JR (1980), Effects of branched chain α-ketoacids on the metabolism of isolated rat liver cells. III. Interactions with pyruvate dehydrogenase. J Biol Chem 255:413–418
Zielke HR, Huang Y, Baab PJ et al (1997) Effect of alpha-ketoisocaproate and leucine on the in vivo oxidation of glutamate and glutamine in the rat brain. Neurochem Res 22:1159–1164
McKenna MC, Sonnewald U, Huang X et al (1998) Alpha-ketoisocaproate alters the production of both lactate and aspartate from [U-13C] glutamate in astrocytes: a 13C NMR study. J Neurochem 70:1001–1008
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” # 01.06.0842-00, and Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Federal do Rio Grande do Sul (PROPESq-UFRGS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro, C.A., Sgaravatti, Â.M., Rosa, R.B. et al. Inhibition of Brain Energy Metabolism by the Branched-chain Amino Acids Accumulating in Maple Syrup Urine Disease. Neurochem Res 33, 114–124 (2008). https://doi.org/10.1007/s11064-007-9423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9423-9