Abstract
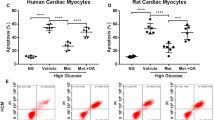
Metformin (MET), an anti-diabetic oral drug with antioxidant properties, has been proved to provide cardioprotective effects in patients with diabetic disease. However, the mechanism is unclear. This study aimd to investigate the effects of MET on the expressions of receptor for advanced glycation end products (RAGE) and high mobility group box 1 protein (HMGB1) in hyperglycemia-treated neonatal rat ventricular myocytes. Cardiocytes were prepared and cultured with high glucose and different concentrations of MET. The expressions of RAGE and HMGB1 were evaluated by Western blot analysis. The superoxide dismutase (SOD), malondialdehyde (MDA), tumor necrosis factor-α (TNF-α), lactate dehydrogenase (LDH) and creatine kinase (CK) were measured. After 12 h-incubation, MET significantly inhibited the increase of MDA, TNF-α, LDH and CK levels induced by high glucose, especially at the 5 × 10−5 to 10−4 mol/L concentrations while inhibiting the decrease of SOD level. Meanwhile, RAGE and HMGB1 expression were significantly increased induced by hyperglycaemia for 24 h (P < 0.05). MET inhibited the expressions of RAGE and HMGB1 in a dose-dependent manner, especially at the 5 × 10−5 to 10−4 mol/L concentrations (P < 0.05). In conclusion, our study suggested that MET could reduce hyperglycemia-induced cardiocytes injury by inhibiting the expressions of RAGE and HMGB1.




Similar content being viewed by others
Abbreviations
- MET:
-
Metformin
- HMGB1:
-
High mobility group box 1 protein
- RAGE:
-
Receptor for advanced glycation end products
- TNF-α:
-
Tumor necrosis factor-α
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- LDH:
-
Lactate dehydrogenase
- CK:
-
Creatine kinase
References
Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan WC, Elliston K, Stern D, Shaw A (1992) Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 267:14998–15004
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Portro A, Bachi A, Rubartelli A, Aqresti A, Bianchi ME (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBOJ 22:5551–5560
Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P (2002) HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine 18:231–236
Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 101:296–301
Volz HC, Seidel C, Laohachewin D, Kaya Z, Müller OJ, Pleger ST, Lasitschka F, Bianchi ME, Remppis A, Bierhaus A, Katus HA, Andrassy M (2010) HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol 105:805–820
Hu X, Jiang H, Bai Q, Zhou X, Xu C, Lu Z, Cui B, Wen H (2009) Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin Chim Acta 406:139–142
Hu X, Zhou X, He B, Xu C, Wu L, Cui B, Wen H, Lu Z, Jiang H (2010) Minocycline protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Eur J Pharmacol 638:84–89
Hu X, Zhou W, Bai Q, Wang J, Yang X, Xu C, Jing H (2011) Increased serum high mobility group box 1 protein in patients with atrial fibrillation. Biomed Aging Pathol 1:52–55
Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM (2004) Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism 53:159–164
UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS34). Lancet 352:854–865
Bonnefont-Rousselot D, Raji B, Walrand S, Gardès-Albert M, Jore D, Legrand A, Peynet J, Vasson MP (2003) An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism 52:586–589
Ouslimani N, Mahrouf M, Peynet J, Bonnefont-Rousselot D, Cosson C, Legrand A, Beaudeux JL (2007) Metformin reduces endothelial cell expression of both the receptor for advanced glycation end products and lectin-like oxidized receptor 1. Metabolism 56:308–313
Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH (2010) Metformin reduces intracellular reactive oxygen species levels by up regulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun 396:199–205
Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC (2011) Metformin inhibits HMGB1 release in LPS-treated RAW264.7cells and increases survival rate of endotoxaemic mice. Br J Pharmacol 162:1498–1508
Yan L, Tang Q, Shen D, Peng S, Zheng Q, Guo H, Jiang M, Deng W (2008) SOCS-1 inhibits TNF-alpha-induced cardiomyocyte apoptosis via ERK1/2 pathway activation. Inflammation 31:180–188
Cai Y, Hu X, Yi B, Zhang T, Wen Z (2012) Glucagon-like peptide-1 protects against hyperglycemia-induced cardiomyocytes injury by inhibiting high group box 1 expression. Mol Biol Rep 39:10705–10711
Hu X, Cui B, Zhou X, Xu C, Lu Z, Jiang H (2012) Ethyl pyruvate reduces myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Mol Biol Rep 39:227–231
Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbäurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A (2008) High-mobility group box-1 in ischemia–reperfusion injury of the heart. Circulation 117:3216–3226
Andrassy M, Volz HC, Bianchi ME (2008) The role of Hmgb1 in the development of diabetic cardiomyopathy. Circulation 118:S351–S352
Bell CW, Jiang W, Reich CF 3rd, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291:C1318–C1325
Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T (2010) The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipo polysaccharide-induced myocardial dysfunction via a TLR4/phosphatidy linositol 3-kinase γ pathway. J Immunol 184:1492–1498
Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S (2009) Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 182:8005–8014
Tan BK, Adya R, Chen J, Farhatullah S, Heutling D, Mitchell D, Lehnert H, Randeva HS (2009) Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res 83:566–574
Gonzalez-Angulo AM, Meric-Bernstam F (2010) Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res 16:1695–1700
Ouslimani N, Peynet J, Bonnefont-Rousselot D, Thérond P, Legrand A, Beaudeux JL (2005) Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 54:829–834
Kalinina N, Aqrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A (2004) Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol 24:2320–2325
Alhaider AA, Korashy HM, Sayed-Anmed MM, Mobark M, Kfoury H, Mansour MA (2011) Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact 192:233–242
Fidan E, Onder Ersoz H, Yilmaz M, Yilmaz H, Kocak M, Karahan C, Erem C (2011) The effects of rosiglitazone and metformin on inflammation and endothelial dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol 48:297–302
Wang XF, Zhang JY, Li L, Zhao XY, Tao HL, Zhang L (2011) Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol 38:94–101
Yao D, Brownlee M (2010) Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 59:249–255
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature 404:787–790
Acknowledgments
This study was partially supported by a grant from National Natural Science foundation of China (No. 81100146 and 81370308), Grant 111023 from the Fundamental Research Funds for the Central Universities and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20110141120060), and the Fundamental Research Funds of Wuhan City (No. 2013070104010044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, T., Hu, X., Cai, Y. et al. Metformin protects against hyperglycemia-induced cardiomyocytes injury by inhibiting the expressions of receptor for advanced glycation end products and high mobility group box 1 protein. Mol Biol Rep 41, 1335–1340 (2014). https://doi.org/10.1007/s11033-013-2979-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2979-3