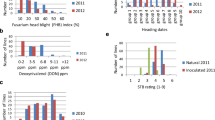
Abstract
Fusarium head blight (FHB) is one of the most economically important wheat diseases, resulting in losses in grain yield and quality as well as contamination with deoxynivalenol (DON). Cultivar Sumai 3 from China and its descendants as well as var. Frontana from Brazil have been identified as potent sources of resistance and subsequently mapped by molecular markers. The aim of the present study was to compare phenotypic and marker-based selection in spring wheat. In a double cross, we combined two donor-quantitative trat loci (QTL) alleles from CM82036 (Sumai 3/Thornbird) located on chromosomes 3B and 5A and one donor-QTL allele from var. Frontana on chromosome 3A with two high-yielding German spring wheat varieties. This initial population was selected phenotypically by a two- (CP1) and three-step procedure (CP1+) and by independent marker-based analysis using one to three flanking markers per QTL (CM). To estimate selection gain, the two phenotypically selected variants and the marker-selected variant as well as an unselected variant (C0) were inoculated with FHB in the field at four locations in 2004. Between 26 and 135 progeny were tested from each variant. FHB severity and DON content were significantly reduced by all selection variants. The highest total selection gain was obtained by the three-step phenotypic selection for both traits, although marker-based selection for the two donor-QTL alleles from CM82036 proved to be more powerful on an annual basis. The large range of variation for FHB resistance and, to a lesser extent, DON content within the marker-based variant, however, shows that an additional phenotypic selection will enhance selection gain.



Similar content being viewed by others
References
Anderson JA, Stack RW, Liu S, Waldron BL, Fjeld AD, Coyne C, Moreno-Sevilla, Mitchell Fetch J, Song QJ, Cregan PB, Frohberg RC (2001) DNA markers for Fusarium head blight resistance QTL in two wheat populations. Theor Appl Genet 102:1164–1168
Bai G, Shaner G (1994) Scab of wheat: prospects for control. Plant Dis 78:760–766
Bai G, Kolb FL, Shaner G, Dornier LL (1999) Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling scab resistance in wheat. Phytopathology 89:343–347
Buerstmayr H, Steiner B, Hartl L, Griesser M, Angerer N, Lengauer D, Miedaner T, Schneider B, Lemmens M (2003) Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat II. Resistance to fungal penetration and spread. Theor Appl Genet 107:503–508
Dudley JW (1993) Molecular markers in plant improvement: manipulation of genes affecting quantitative traits. Crop Sci 33:660–668
Emrich K, Wilde F, Miedaner T, Piepho HP (2007) REML approach for adjusting the Fusarium head blight rating to a phenological date in inoculated selection experiments of wheat. Theor Appl Genet (accepted)
Falconer DS, Mackay FC (1996) Introduction to quantitative genetics, 4th edn. Longman, Essex
Fehr WR (1987) Principles of cultivar development, vol 1: theory and technique. Macmillan, New York
Frisch M, Melchinger AE (2001) Marker-assisted backcrossing for simultaneous introgression of two genes. Crop Sci 41:1716–1725
Frisch M, Bohn M, Melchinger AE (1999) Minimum sample size and optimal positioning of flanking markers in marker-assisted backcrossing for transfer of a target gene. Crop Sci 39:967–975
Hinkelmann K, Kempthorne O (1994) Design and analysis of experiments: introduction to experimental design vol 1. Wiley, New York
Hospital F, Charcosset A (1997) Marker-assisted introgression of quantitative trait loci. Genetics 147:1469–1485
Hospital F, Moreau L, Lacoudre F, Charcosset A, Gallais A (1997) More of the efficiency of marker-assisted selection. Theor Appl Genet 95:1181–1189
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute, Cary, Publications order 55235
Littell RC, Stroup WW, Freund RJ (2002) SAS for linear models, 4th edn. SAS Institute, Cary, N.C.
McMullen M, Jones R, Gallenberg D (1997) Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis 81:1340–1348
Mesterházy Á (1995) Types and components of resistance to Fusarium head blight of wheat. Plant Breed 114:377–386
Mesterházy Á, Barók T, Mirocha CG, Komoróczy R (1999) Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed 118:97–110
Miedaner T, Gang G, Geiger HH (1996) Quantitative-genetic basis of aggressiveness of 42 isolates of Fusarium culmorum for winter rye head blight. Plant Dis 80:500–504
Miedaner T, Reinbrecht C, Lauber U, Schollenberger M, Geiger HH (2001) Effects of genotype and genotype × environment interaction on deoxynivalenol accumulation and resistance to Fusarium head blight in rye, triticale, and wheat. Plant Breed 120:97–105
Miedaner T, Heinrich N, Schneider B, Oettler G, Rohde S, Rabenstein F (2004) Estimation of deoxynivalenol (DON) content by symptom rating and exoantigen content for resistance selection in wheat and triticale. Euphytica 139:123–132
Miedaner T, Wilde F, Steiner B, Buerstmayr H, Korzun V, Ebmeyer E (2006) Stacking quantitative trait loci (QTL) for Fusarium head blight resistance from non-adapted sources in an European elite spring wheat background and assessing their effects on deoxynivalenol (DON) content and disease severity. Theor Appl Genet 112:562–569
Moreau L, Lamarie S, Charcosset A, Gallais A (2000) Economic efficiency of one cycle of marker-assisted selection. Crop Sci 40:329–337
Oettler G, Heinrich N, Miedaner T (2004) Estimates of additive and dominance effects for Fusarium head blight resistance of winter triticale. Plant Breed 123:525–520
SAS Institute (2001) SAS/STAT user’s guide version 802. SAS Institute, Cary, N.C.
Schmolke M, Zimmermann G, Buerstmayr H, Schweizer G, Miedaner T, Korzun V, Ebmeyer E, Hartl L (2005) Molecular mapping of Fusarium head blight resistance in the winter wheat population Dream/Lynx. Theor Appl Genet 111:747–756
Schroeder HW, Christensen JJ (1963) Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 53:831–838
Shen X, Zhou M, Lu W, Ohm H (2003) Detection of Fusarium head blight resistance QTL in a wheat population using bulked segregant analysis. Theor Appl Genet 106:1041–1047
Snijders CHA (1990) Response to selection in F2 generations of winter wheat for resistance to head blight caused by Fusarium culmorum. Euphytica 50:163–169
Steiner B, Lemmens M, Griesser M, Scholz U, Schondelmaier J, Buerstmayr H (2004) Molecular mapping of resistance to Fusarium head blight in the spring wheat cultivar Frontana. Theor Appl Genet 109:214–224
Waldron BL, Moreno-Sevilla B, Anderson JA, Stack RW, Frohberg RC (1999) RFLP mapping of QTL for Fusarium head blight resistance in wheat. Crop Sci 39:805–811
Wilde F, Miedaner T (2006) Selection for Fusarium head blight resistance in early generations reduces deoxynivalenol (DON) content in grain of winter and spring wheat. Plant Breed 125:96–98
Yousef GG, Juvic JA (2001) Comparison of phenotypic and marker-assisted selection for quantitative traits in sweet corn. Crop Sci 41:645–655
Zhou WC, Kolb FL, Bai GH, Domier LL, Boze LK, Smith NJ (2003) Validation of a major QTL for scab resistance with SSR markers and use of marker-assisted selection in wheat. Plant Breed 122:40–46
Acknowledgements
We thank O. Kram, M. Raith, Stefanie Sabrowski, Bianca Schneider, and Meike Scholz for their excellent technical assistance in data collection and Dr. Katharina Emrich, University of Hohenheim, Bioinformatics Group, for her great help with statistical analyses. Special thanks go to Prof. Dr. H. Buerstmayr and Dr. Barbara Steiner, University of Natural Resources and Applied Life Sciences, Institute of Biotechnology in Plant Production, IFA Tulln, Austria, for sharing the resistance donors. This project was supported by the German Federal Ministry of Education and Research (BMBF, Bonn; FKZ 0312559) and the Lochow-Petkus GmbH within the German–French EUREKA Consortium (Project No. Σ! 2386).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilde, F., Korzun, V., Ebmeyer, E. et al. Comparison of phenotypic and marker-based selection for Fusarium head blight resistance and DON content in spring wheat. Mol Breeding 19, 357–370 (2007). https://doi.org/10.1007/s11032-006-9067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-006-9067-5