Abstract
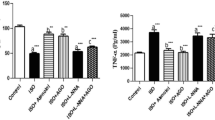
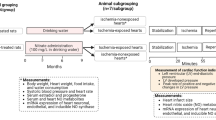
Myocardial infarction leads to a reduction in nitric oxide (NO) bioavailability and an increase in reactive oxygen species (ROS) levels. This scenario has been shown to be detrimental to the heart. Recent studies have shown that thyroid hormone (TH) administration presents positive effects after ischaemic injury. Based on this, the aim of this study was to evaluate the effect of TH on NO bioavailability as well as on endothelial nitric oxide synthase (eNOS) expression after myocardial infarction. Male Wistar rats were divided into three groups: Sham-operated (SHAM), infarcted (AMI) and infarcted + TH (AMIT). During 26 days, the AMIT group received T3 and T4 (2 and 8 µg/100 g/day, respectively) by gavage, while SHAM and AMI rats received saline. After this, the rats underwent echocardiographic analysis were sacrificed, and the left ventricle was collected for biochemical and molecular analysis. Statistical analysis: one-way ANOVA with Student–Newman–Keuls post test. AMI rats presented a 38 % increase in ROS levels. TH administration prevented these alterations in AMIT rats. The AMIT group presented an increase in eNOS expression, in NOS activity and in nitrite levels. TH administration also increased PGC-1α expression in the AMIT group. In conclusion, TH effects seem to involve a modulation of eNOS expression and an improvement in NO bioavailability in the infarcted heart.




Similar content being viewed by others
References
Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB (2001) Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281:1734–1745
Singh N, Dhalla AK, Seneviratne C, Singal PK (1995) Oxidative stress and heart failure. Mol Cell Biochem 147:77–81
Schenkel PC, Tavares AM, Fernandes RO, Diniz GP, Ludke AR, Ribeiro MF, Araujo AS, Barreto-Chaves ML, Belló-Klein A (2012) Time profile of hydrogen peroxide/thioredoxin balance and its influence in the intracellular signaling post-myocardial infarction. Exp Physiol 97:741–749
Schenkel PC, Tavares AM, Fernandes RO, Diniz GP, Bertagnolli M, da Rosa Araujo AS, Barreto-Chaves ML, Ribeiro MF, Clausell N, Belló-Klein A (2010) Redox-sensitive prosurvival and proapoptotic protein expression in the myocardial remodeling post-infarction in rats. Mol Cell Biochem 341:1–8
Berndt C, Lillig CH, Holmgren A (2007) Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol 292:1227–1236
Tao L, Gao E, Hu A, Coletti C, Wang Y, Christopher TA, Lopez BL, Koch W, Ma XL (2006) Thioredoxin reduces post-ischemic myocardial apoptosis by reducing oxidative/nitrative stress. Br J Pharmacol 149:311–318
Lind C, Gerdes R, Schuppe-Koistinen I, Cotgreave IA (1998) Studies on the mechanism of oxidative modification of human glyceraldehyde-3-phosphate dehydrogenase by glutathione: catalysis by glutaredoxin. Biochem Biophys Res Commun 247:481–486
Barett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB (1999) Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38:6699–6705
Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G (1999) Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 100:292–298
Heusch G, Post H, Michel MC, Kelm M, Schulz R (2000) Endogenous nitric oxide and myocardial adaptation to ischemia. Circ Res 87:146–152
Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vançon AC, Huang PL, Lee RT, Zapol WM, Picard MH (2001) Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104:1286–1291
Shi CZ, Zhang XP, Lv ZW, Zhang HL, Xu JZ, Yin ZF, Yan YQ, Wang CQ (2012) Adipose tissue-derived stem cells embedded with eNOS restore cardiac function in acute myocardial infarction model. Int J Cardiol 154:2–8
Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899
Forini F, Lionetti V, Ardehali H, Pucci A, Cecchetti F, Ghanefar M, Nicolini G, Ichikawa Y, Nannipieri M, Recchia FA, Iervasi G (2011) Early long-term L-T3 replacement rescues mitochondria and prevents ischemic cardiac remodelling in rats. J Cell Mol Med 15:514–524
Pantos C, Mourouzis I, Markakis K, Tsagoulis N, Panagiotou M, Cokkinos DV (2008) Long-term thyroid hormone administration reshapes left ventricular chamber and improves cardiac function after myocardial infarction in rats. Basic Res Cardiol 103:308–318
Mourouzis I, Giagourta I, Galanopoulos G, Mantzouratou P, Kostakou E, Kokkinos AD, Tentolouris N, Pantos C (2013) Thyroid hormone improves the mechanical performance of the post-infarcted diabetic myocardium: a response associated with up-regulation of Akt/mTOR and AMPK activation. Metabolism 62:1387–1393
De Castro AL, Tavares AMV, Campos C, Fernandes RO, Siqueira R, Conzatti A, Bicca AM, Fernandes TR, Sartório CL, Schenkel PC, Belló-Klein A, da Rosa Araujo AS (2014) Cardioprotective effects of thyroid hormones in a rat model of myocardial infarction are associated with oxidative stress reduction. Mol Cell Endocrinol 391:22–29
Johns TNP, Olson BJ (1954) Experimental myocardial infarction: a method of coronary occlusion in small animals. Ann Surg 140:675–682
Araujo AS, Ribeiro MF, Enzveiler A, Schenkel P, Fernandes TR, Partata WA, Irigoyen MC, Llesuy S, Belló-Klein A (2006) Myocardial antioxidant enzyme activities and concentration and glutathione metabolism in experimental hyperthyroidism. Mol Cell Endocrinol 249:133–139
Peron AP, Saraiva RM, Antonio EL, Tucci PJ (2006) Mechanical function is normal in remanent myocardium during the healing period of myocardial infarction despite congestive heart failure. Arq Bras Cardiol 86:105–112
Tavares AM, da Rosa Araújo AS, Baldo G, Matte U, Khaper N, Belló-Klein A, Rohde LE, Clausell N (2010) Bone marrow derived cells decrease inflammation but not oxidative stress in an experimental model of acute myocardial infarction. Life Sci 87:699–706
Llesuy SF, Milei J, Molina H, Boveris A, Milei S (1985) Comparison of lipid peroxidation and myocardial damage induced by adramiycin and 4′-epiadramiycin in mice. Tumori 71:241–249
Lebel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Holmgren A, Aslund F (1995) Glutaredoxin. Methods Enzymol 252:283–292
Holmegren A, Björnstedt M (1995) Thioredoxin and thioredoxin reductase. Methods Enzymol 252:199–208
Valdez LB, Zaobornyj T, Boveris A (2005) Functional activity of mitochondrial nitric oxide synthase. Methods Enzymol 396:444–455
Granger DL, Anstey NM, Miller WC, Weinberg JB (1999) Measuring nitric oxide production in human clinical studies. Methods Enzymol 301:49–61
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Klein D, Kern RM, Sokol RZ (1995) A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36:59–66
Olivares EL, Marassi MP, Fortunato RS, da Silva AC, Costa-e-Sousa RH, Araújo IG, Mattos EC, Masuda MO, Mulcahey MA, Huang SA, Bianco AC, Carvalho DP (2007) Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology 148:4786–4792
Pingitore A, Iervasi G (2008) Triiodothyronine (T3) effects on cardiovascular system in patients with heart failure. Recent Pat Cardiovasc Drug Discov 3:19–27
Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56:56–64
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Pineda-Molina E, Klatt P, Vazquez J, Martina A, de Lacoba MG, Perez-Sala D, Lamas S (2011) Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40:14134–14142
Strauer BE, Beer K, Heitlinger K, Höfling B (1977) Left ventricular systolic wall stress as a primary determinant of myocardial oxygen consumption: comparative studies in patients with normal left ventricular function, with pressure and volume overload and with coronary heart disease. Basic Res Cardiol 72:306–313
Araujo AS, Diniz G, Seibel F, Branchini G, Ribeiro MF, Brum IS, Khaper N, Barreto-Chaves ML, Belló-Klein A (2011) Reactive oxygen and nitrogen species balance in the determination of thyroid hormones-induced cardiac hypertrophy mediated by Renin-Angiotensin system. Mol Cell Endocrinol 333:78–84
Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, Colucci WS, Sutton MG, Selwyn AP, Alexander RW et al (1999) Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation 81:772–779
Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714
Spooner PH, Thai HM, Goldman S, Gaballa MA (2004) Thyroid hormone analog, DITPA, improves endothelial nitric oxide and beta-adrenergic mediated vasorelaxation after myocardial infarction. J Cardiovasc Pharmacol 44:453–459
Cote CG, Yu FS, Zulueta JJ, Vosatka RJ, Hassoun PM (1996) Regulation of intracellular xanthine oxidase by endothelial-derived nitric oxide. Am J Physiol 271:869–874
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1αlpha. Cardiovasc Res 79:208–217
Fernandes RO, Bonetto JH, Baregzay B, de Castro AL, Puukila S, Forsyth H, Schenkel PC, Llesuy SF, Brum IS, Araujo AS, Khaper N, Belló-Klein A (2015) Modulation of apoptosis by sulforaphane is associated with PGC-1α stimulation and decreased oxidative stress in cardiac myoblasts. Mol Cell Biochem 401:61–70
Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B (2010) PGC-1α regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 13:1011–1022
Vallejo CG, Seguido AM, Testillano PS, Risueño MC (2005) Thyroid hormone regulates tubulin expression in mammalian liver. Effects of deleting thyroid hormone receptor-α or –β. Am J Physiol Endocrinol Metab 289:1
Samuel JL, Marotte F, Delcayre C, Rappaport L (1986) Microtubule reorganization is related to rate of heart myocyte hypertrophy in rat. Am J Physiol 251:1118–1125
Acknowledgments
This work was supported by the Brazilian Research Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS). We are indebted to Dr. Constantinos Pantos and to Dr. Iordanis Mourouzis for their full assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest. The authors declare that the experimental procedures were performed taking into consideration the welfare of animals and were approved by the Ethics Committee for animal research at Universidade Federal do Rio Grande do Sul.
Rights and permissions
About this article
Cite this article
de Castro, A.L., Tavares, A.V., Fernandes, R.O. et al. T3 and T4 decrease ROS levels and increase endothelial nitric oxide synthase expression in the myocardium of infarcted rats. Mol Cell Biochem 408, 235–243 (2015). https://doi.org/10.1007/s11010-015-2501-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2501-4