Abstract
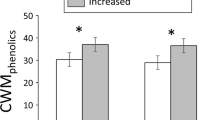
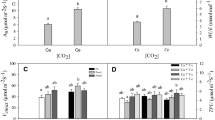
Global climate change includes concomitant changes in many components of the abiotic flux necessary for plant life. In this paper, we investigate the combined effects of elevated CO2 (720 ppm) and temperature (+2 K) on the phytochemistry of three deciduous tree species. The analysis revealed that elevated CO2 generally stimulated increased carbon partitioning to various classes of phenolic compounds, whereas an increase in temperature had the opposite effect. The combined effects of both elevated CO2 and temperature were additive, i.e., canceling one another’s individual effects. Obviously, the effects of global climate change on leaf chemistry must simultaneously consider both temperature and CO2. If these results are generally applicable, then the counteracting effect of the temperature is likely to play a major role in alpine, boreal, and arctic zones in determining the balance between populations of plants and herbivores.





Similar content being viewed by others
References
Agrell, J., Anderson, P., Oleszek, W., Stochmal, A. and Agrell, C. 2004. Combined effects of elevated CO2 and herbivore damage on alfalfa and cotton. J. Chem. Ecol. 30:2309–2324.
Battisti, A., Stastny, M., Netherer, S., Robinet, C., Schopf, A., Roques, A., and Larsson, S. 2005. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol. Appl. 15:2084-2096.
Bezemer, T. M. and Jones, T. H. 1998. Plant–insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos 82:212–222.
Haase, D. L. and Rose, R. 1995. Vector analysis and its use for interpreting plant nutrient shifts in response to silvicultural treatments. Forest Sci. 41:54–66.
Hall, M. C., Stiling, P., Moon, D. C., Drake, B. G. and Hunter, M. D. 2005. Effects of elevated CO2 on foliar quality and herbivore damage in a scrub oak ecosystem. J. Chem. Ecol. 31:267–286.
Herms, D. A. 2002. Effects of fertilization on the insect resistance of ornamental woody plants: reassessing an entrenched paradigm. Environ. Entomol. 31:923–933.
Herms, D. A. and Mattson, W. J. 1992. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67:283–335.
Hunter, M. D. 2001. Effects of elevated atmospheric carbon dioxide on insect–plant interactions. Agric. For. Entomol. 3:153–159.
Holopainen, J. K. and Kainulainen, P. 2004. Reproductive capacity of the grey pine aphid and allocation response of Scots pine seedlings across temperature gradients: a test of hypotheses predicting outcomes of global warming. Can. J. For. Res. 34:94–102.
IPCC 1996. Climate Change 1995: The Science of Climate Change. Cambridge University Press, Cambridge.
Johnson, R. H. and Lincoln, D. E. 1991. Sagebrush carbon allocation patterns and grasshopper nutrition: the influence of CO2 enrichment and soil mineral limitation. Oecologia 87:127–134.
Kellomäki, S. and Wang, K.-Y. 1998. Sap flow in Scots pines growing under conditions of year-round carbon dioxide enrichment and temperature elevation. Plant Cell Environ. 2:969–981.
Kellomäki, S.,Wang, K.-Y. and Lemettinen, M. 2000. Controlled environment chambers for investigating tree response to elevated CO2 and temperature under boreal conditions. Photosynthetica 38:69–81.
Koricheva, J. 1999. Interpreting phenotypic variation in plant allelochemistry: problems with the use of concentrations. Oecologia 119:467–473.
Kuokkanen, K., Julkunen-Tiitto, R., Keinänen, M., Niemelä, P. and Tahvanainen, J. 2001. The effect of elevated CO2 and temperature on the secondary chemistry of Betula pendula seedlings. Trees 15:378-384.
Kuokkanen, K., Yan, P. and Niemelä, P. 2003. Effects of elevated CO2 and temperature on the leaf chemistry of Betula pendula (Roth) and the feeding behaviour of the weevil Phyllobius maculicornis. Agric. For. Entomol. 5:209–217.
Kuokkanen, K., Niemelä, P., Matala, J., Julkunen-Tiitto, R., Heinonen, J., Rousi, M., Henttonen, H., Tahvanainen, J. and Kellomäki, S. 2004. The effects of elevated CO2 and temperature on the resistance of winter-dormant birch seedlings to hares and voles. Glob. Chang. Biol. 10:1504–1512.
Lavola, A. and Julkunen-Tiitto, R. 1994. The effect of elevated carbon dioxide and fertilization on primary and secondary metabolites in birch, Betula pendula (Roth). Oecologia 99, 315–321.
Lavola, A., Julkunen-Tiitto, R., Roininen, H. and Aphalo, P. 1998. Host-plant preference of an insect herbivore mediated by UV-B and CO2 in relation to plant secondary metabolites. Biochem. Syst. Ecol. 26:1–12.
Lincoln, D. E., Fajer, E. D., and Johnson, R. H. 1993. Plant–insect herbivore interactions in elevated CO2 environments. Trends Ecol. Evol. 8:64–68.
Lindroth, R. L., Kinney, K. K. and Platz, C. L. 1993. Responses of deciduous trees to elevated atmospheric CO2: productivity, phytochemistry and insect performance. Ecology 74:763–777.
Lindroth, R. L., Klein, K. A., Hemming, J. D. C. and Feuker, A. M. 1997. Variation in temperature and dietary nitrogen affect performance of the gypsy moth (Lymantria dispar L.). Phys. Entomol. 22:55–64.
Loladze, I. 2002. Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 17:457–461.
Mattson, W. J. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11:119–161.
Mattson, W. J. and Haack, R. A. 1987a. The role of drought in outbreaks of plant-eating insects. BioScience 37:110–118.
Mattson, W. J. and Haack, R. A. 1987b. The role of drought stress in provoking outbreaks of phytophagous insects, pp. 365–407. in Barbosa, P. and Schultz, J.C. (eds.). Insect Outbreaks, Academic Press, NewYork.
Mattson, W. J., Julkunen-Tiitto, R., and Herms, D. A. 2005. CO2 enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition model or growth differentiation model. Oikos 111:337–347.
Parmesan, C., Ryrholm, N., Stefanescu, C., Hill, J. K., Thomas, C. D., Descimon, H., Huntley, B., Kaila, L., Kullberg, J., Tammaru, T., Tennent, W. J., Thomas, J. A. and Warren, M. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583.
Prentice, I. C., Cramer, W., Harrison, S. P., Leemans, R., Monserud, R. A. and Solomon, A. M. 1992. A global biome model based on plant physiology and dominance, soil properties, and climate. J. Biogeogr. 19:117–134.
Reavey, D. 1993. Why body size matters to caterpillars, pp. 248–279, in N. E. Stamp and T. M. Casey (eds.). Caterpillars: Ecological and Evolutionary Constraints on Foraging. Chapman & Hall, New York.
Sterner, R. W. and Elser, J. J. 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton.
Timmer, V. R. and Stone, E. L. 1978. Comparative foliar analysis of young balsam fir fertilized with nitrogen, phosphorus, potassium and lime. Soil Sci. Soc. Am. J. 42:125–130.
Veteli, T. O. 2003. Global atmospheric change and herbivory: effects of elevated levels of UV-B radiation, atmospheric CO2 and temperature on boreal woody plants and their herbivores. PhD Dissertations in Biology No: 19. University of Joensuu, Joensuu.
Veteli, T. O., Kuokkanen, K., Julkunen-Tiitto, R., Roininen, H. and Tahvanainen, J. 2002. Effects of elevated CO2 and temperature on plant growth and herbivore defensive chemistry. Glob. Chang. Biol. 8:1240–1252.
Zvereva, E. L. and Kozlov, M. V. 2006. Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a metaanalysis. Glob. Change Biol. 12:27–41.
Acknowledgments
We thank Dr. Marja-Leena Laitinen for valuable hints. We are also grateful to Dr. John Derome for checking the language of this article. This study was supported by the Academy of Finland (Finnish Centre of Excellence Program for Forest Ecology and Management 2000–2005, project no. 64308 and 51997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veteli, T.O., Mattson, W.J., Niemelä, P. et al. Do Elevated Temperature and CO2 Generally Have Counteracting Effects on Phenolic Phytochemistry of Boreal Trees?. J Chem Ecol 33, 287–296 (2007). https://doi.org/10.1007/s10886-006-9235-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9235-4