Abstract
Erlotinib (ERL) is a drug used in epidermoid carcinoma treatment. One of the ERL drawbacks is low water solubility, which limits its use in the development of safer and effective formulations. The present study used the strategy of inclusion complexation with hydroxypropyl-β-cyclodextrin (HP-β-CD) to increase the ERL water solubility, characterize the inclusion complex physico-chemically, and evaluate the in vitro cytotoxicity and in vivo antiangiogenic effect. The data showed 1:1 molar ratio ERL:HP-β-CD inclusion complex formation both in solution and in solid state. Phase-solubility analysis showed AL-type diagrams. Isothermal titrations calorimetry and nuclear Overhauser effect spectroscopy also support that formation. Despite free ERL higher cytotoxicity, higher values were associated with the complex compared with free ERL (37.5 µM), and the complex was more cytotoxic to A431 human epidermoid carcinoma cell than to osteoblasts (non-cancerous cells). In addition, the inclusion complex exhibited antiangiogenic activity without affecting the activation and recruitment of neutrophils and macrophage. Overall, these results suggest that the ERL:HP-β-CD inclusion complex could be a promising approach for developing safe and effective ERL formulation by different routes of administration.











Similar content being viewed by others
References
Soulieres, D., Senzer, N.N., Vokes, E.E., Hidalgo, M., Agarwala, S.S., Siu, L.L.: Multicenter phase ii study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J. Clin. Oncol. 22, 77–85 (2004)
Kralj, S., Rojnik, M., Kos, J., Makovec, D.: Targeting egfr-overexpressed a431 cells with egf-labeled silica-coated magnetic nanoparticles. J. Nanopart. Res. 15, 11 (2013)
Schneider, M.R., Sibilia, M., Erben, R.G.: The egfr network in bone biology and pathology. Trends Endocrinol. Metab. 20, 517–524 (2009)
Mahller, Y.Y., Vaikunth, S.S., Currier, M.A., Miller, S.J., Ripberger, M.C., Hsu, Y.H., Mehrian-Shai, R., Collins, M.H., Crombleholme, T.M., Ratner, N., Cripe, T.P.: Oncolytic hsv and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol. Ther. 15, 279–286 (2007)
Ding, G., Liu, F., Feng, C., Xu, J., Ding, Q.: Association between the myeloperoxidase gene polymorphisms and the susceptibility to prostate cancer: a case-control study in a chinese population. Actas Urol. Esp. 37, 79–82 (2013)
Tucker, S.M., Pierce, R.J., Price, R.G.: Characterization of human n-acetyl-beta-d-glucosaminidase isoenzymes as an indicator of tissue-damage in disease. Clin. Chim. Acta 102, 29–40 (1980)
Balkwill, F.: Tnf-alpha in promotion and progression of cancer. Cancer Metast. Rev. 25, 409–416 (2006)
Coelho, B.A., Belo, A.V., Andrade, S.P., Amorim, W.C., Uemura, G., da Silva, A.L.: N-acetylglucosaminidase, myeloperoxidase and vascular endothelial growth factor serum levels in breast cancer patients. Biomed. Pharmacother. 68, 185–189 (2014)
Cohen, E.E.W., Halpern, A.B., Kasza, K., Kocherginsky, M., Williams, R., Vokes, E.E.: Factors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 45, E155–E160 (2009)
Fan, L., Hu, L., Yang, B., Fang, X., Gao, Z., Li, W., Sun, Y., Shen, Y., Wu, X., Shu, Y., Gu, Y., Wu, X., Xu, Q.: Erlotinib promotes endoplasmic reticulum stress-mediated injury in the intestinal epithelium. Toxicol. Appl. Pharmacol. 278, 45–52 (2014)
Herchenhorn, D., Dias, F.L., Viegas, C.M.P., Federico, M.H., Araujo, C.M.M., Small, I., Bezerra, M., Fontao, K., Knust, R.E., Ferreira, C.G., Martins, R.G.: Phase i/ii study of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 78, 696–702 (2010)
Thomas, S.M., Grandis, J.R.: Pharmacokinetic and pharmacodynamic properties of egfr inhibitors under clinical investigation. Cancer Treat. Rev. 30, 255–268 (2004)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Venturini, C.D.G., Nicolini, J., Machado, C., Machado, V.G.: Properties and recent applications of cyclodextrins. Quim. Nova 31, 360–368 (2008)
Khan, A.R., Forgo, P., Stine, K.J., D’Souza, V.T.: Methods for selective modifications of cyclodextrins. Chem. Rev. 98, 1977–1996 (1998)
Gould, S., Scott, R.C.: 2-hydroxypropyl-beta-cyclodextrin (hp-beta-cd): a toxicology review. Food Chem. Toxicol. 43, 1451–1459 (2005)
Yan, J., Li, Q.F., Wang, L.S., Wang, H., Xiao, F.J., Yang, Y.F., Wu, C.T.: Methyl-beta-cyclodextrin induces programmed cell death in chronic myeloid leukemia cells and combined with imatinib, produces a synergistic downregulation of erk/spk1 signaling. Anticancer Drugs 23, 22–31 (2012)
Lee, Y.H.P., Sathigari, S., Lin, Y.J.J., Ravis, W.R., Chadha, G., Parsons, D.L., Rangari, V.K., Wright, N., Babu, R.J.: Gefitinib-cyclodextrin inclusion complexes: physico-chemical characterization and dissolution studies. Drug Dev. Ind. Pharm. 35, 1113–1120 (2009)
Kim, C., Shah, B.P., Subramaniam, P., Lee, K.-B.: Synergistic induction of apoptosis in brain cancer cells by targeted codelivery of sirna and anticancer drugs. Mol. Pharm. 8, 1955–1961 (2011)
Suárez, D.F., Consuegra, J., Trajano, V.C., Gontijo, S.M.L., Guimarães, P.P.G., Cortés, M.E., Denadai, Â.L., Sinisterra, R.D.: Structural and thermodynamic characterization of doxycycline/β-cyclodextrin supramolecular complex and its bacterial membrane interactions. Colloids Surf. B 118, 194–201 (2014)
Gontijo, S.M.L., Gomes, A.D.M., Gala-Garcia, A., Sinisterra, R.D., Cortes, M.E.: Evaluation of antimicrobial activity and cell viability of aloe vera sponges. Electron. J. Biotechnol. 16, 10 (2013)
Guimaraes, Goulart, Guimaraes, Goulart, Guimaraes, Goulart, Guimaraes, Goulart, Guimaraes, Goulart, Guimaraes, P.P.G., Oliveira, S.R., de Castro Rodrigues, G., Gontijo, S.M.L., Lula, I.S., Cortes, M.E., Leite Denadai, A.M., Sinisterra, R.D.: Development of sulfadiazine-decorated plga nanoparticles loaded with 5-fluorouracil and cell viability. Molecules 20, 879–899 (2015)
Lobaina, T., Zhurbenko, R., Alfonso, I., Rodriguez, C., Gala-Garcia, A., Gontijo, S.L., Cortes, M.E., Gomes, A., Sinisterra, R.D.: Efficacy of coral-hydroxyapatite and biphasic calcium phosphate for early bacterial detection. Biointerphases 9, 7 (2014)
Bax, A., Davis, D.G.: Practical aspects of two-dimensional transverse noe spectroscopy. J. Magn. Reson. 63, 207–213 (1985)
Denadai, A.M.L., Santoro, M.M., Da Silva, L.H., Viana, A.T., Santos, R.A.S., Sinisterra, R.D.: Self-assembly characterization of the beta-cyclodextrin and hydrochlorothiazide system: Nmr, phase solubility, itc and qels. J. Incl. Phenom. Macrocycl. Chem. 55, 41–49 (2006)
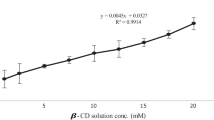
Higuchi, T., Connors, K.A.: Phase solubility techniques. Adv. Anal. Chem. Instr. 4, 117–212 (1965)
Qiu, N., Cheng, X., Wang, G.C., Wang, W.W., Wen, J.L., Zhang, Y.K., Song, H., Ma, L., Wei, Y.Q., Peng, A.H., Chen, L.J.: Inclusion complex of barbigerone with hydroxypropyl-beta-cyclodextrin: preparation and in vitro evaluation. Carbohydr. Polym. 101, 623–630 (2014)
Du, H.Y., Xu, B., Wu, C.X., Li, M., Ran, F.X., Cai, S.Q., Cui, J.R.: Effects of cs-1 on a431 cell proliferation, cell cycle, and epidermal growth factor receptor signal transduction. Acta Biochim. Biophys. Sin. 44, 136–146 (2012)
Ling, Y.H., Li, T.H., Yuan, Z.Q., Haigentz, M., Weber, T.K., Perez-Soler, R.: Erlotinib, an effective epidermal growth factor receptor tyrosine kinase inhibitor, induces p27(kip1) up-regulation and nuclear translocation in association with cell growth inhibition and g1/s phase arrest in human non-small-cell lung cancer cell lines. Mol. Pharmacol. 72, 248–258 (2007)
Iacovino, R., Caso, J.V., Rapuano, F., Russo, A., Isidori, M., Lavorgna, M., Malgieri, G., Isernia, C.: Physicochemical characterization and cytotoxic activity evaluation of hydroxymethylferrocene:beta-cyclodextrin inclusion complex. Molecules 17, 6056–6070 (2012)
Mendes, J.B., Rocha, M.A., Araujo, F.A., Moura, S.A.L., Ferreira, M., Andrade, S.P.: Differential effects of rolipram on chronic subcutaneous inflammatory angiogenesis and on peritoneal adhesion in mice. Microvasc. Res. 78, 265–271 (2009)
Castro, P.R., Marques, S.M., Campos, P.P., Cardoso, C.C., Sampaio, F.P., Ferreira, M., Andrade, S.P.: Kinetics of implant-induced inflammatory angiogenesis in abdominal muscle wall in mice. Microvasc. Res. 84, 9–15 (2012)
Marques, S.M., Campos, P.P., Castro, P.R., Cardoso, C.C., Ferreira, M., Andrade, S.P.: Genetic background determines mouse strain differences in inflammatory angiogenesis. Microvasc. Res. 82, 246–252 (2011)
Boehrer, S., Ades, L., Braun, T., Galluzzi, L., Grosjean, J., Fabre, C., Le Roux, G., Gardin, C., Martin, A., de Botton, S., Fenaux, P., Kroemer, G.: Erlotinib exhibits antineoplastic off-target effects in aml and mds: a preclinical study. Blood 111, 2170–2180 (2008)
Teixeira, A.S., Araujo, F.A., Ferreira, M., Barcelos, L.S., Teixeira, M.M., Andrade, S.P.: Angiogenesis and inflammation in skeletal muscle in response to ascites tumor in mice. Life Sci. 78, 1637–1645 (2006)
Garnero, C., Zoppi, A., Genovese, D., Longhi, M.: Studies on trimethoprim:hydroxypropyl-beta-cyclodextrin: aggregate and complex formation. Carbohydr. Res. 345, 2550–2556 (2010)
Passos, J.J., De Sousa, F.B., Mundim, I.M., Bonfim, R.R., Melo, R., Viana, A.F., Stolz, E.D., Borsoi, M., Rates, S.M.K., Sinisterra, R.D.: In vivo evaluation of the highly soluble oral beta-cyclodextrin-sertraline supramolecular complexes. Int. J. Pharm. 436, 478–485 (2012)
Tien, Y.C., Su, C.S., Lien, L.H., Chen, Y.P.: Recrystallization of erlotinib hydrochloride and fulvestrant using supercritical antisolvent process. J. Supercrit. Fluids 55, 292–299 (2010)
Veiga, F.J.B., Fernandes, C.M., Carvalho, R.A., Geraldes, C.: Molecular modelling and h-1-nmr: ultimate tools for the investigation of tolbutamide:Beta-cyclodextrin and tolbutamide:Hydroxypropyl-beta-cyclodextrin complexes. Chem. Pharm. Bull. 49, 1251–1256 (2001)
Kim, J.H., Lee, S.K., Ki, M.H., Choi, W.K., Ahn, S.K., Shin, H.J., Hong, C.I.: Development of parenteral formulation for a novel angiogenesis inhibitor, ckd-732 through complexation with hydroxypropyl-beta-cyclodextrin. Int. J. Pharm. 272, 79–89 (2004)
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1357 (1997)
Patel, R.P., Patel, M.M.: Preparation and evaluation of inclusion complex of the lipid lowering drug lovastatin with β-cyclodextrin. Dhaka Univ. J. Pharm. Sci. 6, 25–36 (2007)
Turnbull, W.B., Daranas, A.H.: On the value of c: can low affinity systems be studied by isothermal titration calorimetry? J. Am. Chem. Soc. 125, 14859–14866 (2003)
Rekharsky, M.V., Inoue, Y.: Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1917 (1998)
de Paula, W.X., Denadai, A.M.L., Santoro, M.M., Braga, A.N.G., Santos, R.A.S., Sinisterra, R.D.: Supramolecular interactions between losartan and hydroxypropyl-beta-cd: esi mass-spectrometry, nmr techniques, phase solubility, isothermal titration calorimetry and anti-hypertensive studies. Int. J. Pharm. 404, 116–123 (2011)
Rekharsky, M., Inoue, Y., Tobey, S., Metzger, A., Anslyn, E.: Ion-pairing molecular recognition in water: aggregation at low concentrations that is entropy-driven. J. Am. Chem. Soc. 124, 14959–14967 (2002)
Bliesath, J., Huser, N., Omori, M., Bunag, D., Proffitt, C., Streiner, N., Ho, C., Siddiqui-Jain, A., O’Brien, S.E., Lim, J.K.C., Ryckman, D.M., Anderes, K., Rice, W.G., Drygin, D.: Combined inhibition of egfr and ck2 augments the attenuation of pi3k-akt-mtor signaling and the killing of cancer cells. Cancer Lett. 322, 113–118 (2012)
Kim, J.C., Ali, M.A., Nandi, A., Mukhopadhyay, P., Choy, H., Cao, C., Saha, D.: Correlation of her1/egfr expression and degree of radiosensitizing effect of the her1/egfr-tyrosine kinase inhibitor erlotinib. Indian J. Biochem. Biophys. 42, 358–365 (2005)
Li, D.D., Fang, F., Li, J.R., Du, Q.R., Sun, J., Gong, H.B., Zhu, H.L.: Discovery of 6-substituted 4-anilinoquinazolines with dioxygenated rings as novel egfr tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 22, 5870–5875 (2012)
Yamasaki, F., Zhang, D.W., Bartholomeusz, C., Sudo, T., Hortobagyi, G.N., Kurisu, K., Ueno, N.T.: Sensitivity of breast cancer cells to erlotinib depends on cyclin-dependent kinase 2 activity. Mol. Cancer Ther. 6, 2168–2177 (2007)
Dehelean, C.A., Soica, C., Peev, C., Ciurlea, S., Feflea, S., Kasa, P.: A pharmaco-toxicological evaluation of betulinic acid mixed with hydroxipropilgamma cyclodextrin on in vitro and in vivo models. Farmacia 59, 51–59 (2011)
Hovgaard, L.: Drug-delivery studies in caco-2 monolayers.4. Absorption enhancer effects of cyclodextrins. Pharm. Res. 12, 1328–1332 (1995)
Irie, T., Uekama, K.: Cyclodextrins in peptide and protein delivery. Adv. Drug Deliv. Rev. 36, 101–123 (1999)
Ohtani, Y., Irie, T., Uekama, K., Fukunaga, K., Pitha, J.: Differential-effects of alpha-cyclodextrins, beta-cyclodextrins and gamma-cyclodextrins on human-erythrocytes. Eur. J. Biochem. 186, 17–22 (1989)
Björkelund, H., Gedda, L., Malmqvist, M., Andersson, K.: Resolving the egf-egfr interaction characteristics through a multiple-temperature, multiple-inhibitor, real-time interaction analysis approach. Mol. Clin. Oncol. 1, 343–352 (2013)
Folkman, J.: Endogenous angiogenesis inhibitors. Apmis 112, 496–507 (2004)
Yano, H., Hirayama, F., Arima, H., Uekama, K.: Prednisolone-appended alpha-cyclodextrin: alleviation of systemic adverse effect of prednisolone after intracolonic administration in 2,4,6-trinitrobenzenesulfonic acid-induced colitis rats. J. Pharm. Sci. 90, 2103–2112 (2001)
Acknowledgments
We are grateful for CAPES, CNPq, FINEP, FAPEMIG, PRPq, INCT/Nanobiofar, CNPq Nanofar network that support this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gontijo, S.M.L., Guimarães, P.P.G., Viana, C.T.R. et al. Erlotinib/hydroxypropyl-β-cyclodextrin inclusion complex: characterization and in vitro and in vivo evaluation. J Incl Phenom Macrocycl Chem 83, 267–279 (2015). https://doi.org/10.1007/s10847-015-0562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0562-3