Abstract
Beta-glucans are polysaccharides that can be used for different applications, for example as an immunomodulator in food or feed or for managing high cholesterol levels. Certain microalgae species use beta-glucans as energy storage, accumulating them during nutrient depletion. In this study, we examined and compared beta-glucan production during nitrogen depletion in three different algae species, Phaeodactylum tricornutum, Monodopsis subterranea and Cylindrotheca fusiformis, grown in artificially illuminated flat panel airlift reactors, in order to determine the most promising microalgae species for beta-glucan production. Co-products such as fatty acids (especially eicosapentaenoic acid) and the carotenoid fucoxanthin (not produced by M. subterranea) were also considered. Biomass analysis showed that P. tricornutum cultures reached a maximal beta-glucan content of 317 ± 9 mg gDW−1, M. subterranea cultures reached 188 ± 6 mg gDW−1 and C. fusiformis cultures reached 129 ± 13 mg gDW−1. Furthermore, beta-glucan production was faster in P. tricornutum cultures. However, the maximum volumetric beta-glucan concentration reached was higher in M. subterranea cultures compared to P. tricornutum cultures as M. subterranea cultures produced more biomass during nitrogen depletion. In terms of possible co-products, P. tricornutum produced fucoxanthin and EPA, whereas M. subterranea did not produce fucoxanthin. However, M. subterranea exhibited a higher EPA content, which remained above 45 mg g−1 even after several days of nitrogen depletion. Overall, our results suggest that P. tricornutum and M. subterranea are both suitable species for beta-glucan production in flat panel airlift reactors.
Similar content being viewed by others
Introduction
Beta-glucans (ß-glucans) can be found in human diets in the form of yeast or cereals (Ciecierska et al. 2019). Additionally, various products containing ß-glucans are available on the market. For instance, ß-glucans from yeast are sold as animal feed (MacroGard™, Offra). Macro- and microalgae also contain ß-glucans and are therefore possible alternative sources (Myklestad 1989; Aziz et al. 2003).
Microalgae produce ß-glucans in different forms, for example as unbranched 1,3-ß-glucan (paramylon) or as branched 1,3–1,6-ß-glucan (chrysolaminarin). These structural differences affect solubility. Chrysolaminarin is found dissolved in the cytosol, while paramylon is found undissolved as granules (Kiss et al. 1988; Espinoza-Gallardo et al. 2017; Gruber and Kroth 2017). All three microalgae species examined here, Phaeodactylum tricornutum, Monodopsis subterranea and Cylindrotheca fusiformis, are known to accumulate ß-glucans as carbon and energy storage during nutrient depletion. However, P. tricornutum and C. fusiformis produce water-soluble branched chrysolaminarin, while M. subterranea produces unbranched paramylon (Vieler et al. 2012; Eliáš et al. 2017; Gao et al. 2017). Besides carbohydrates, microalgae also accumulate triglycerides as carbon and energy storage during nutrient depletion. These triglycerides contain saturated and unsaturated fatty acids with different chain lengths, such as C16:0, C16:1 and C18:1 (Yodsuwan et al. 2017; Adamakis et al. 2018). Therefore, the accumulation of these fatty acids, which are used for energy storage, must be taken into account when examining the production of ß-glucans.
Various possible applications have been described for the use of algae-derived ß-glucans, for example in food, feed or agriculture. It has been reported that ß-glucans show anti-oxidative, immunomodulatory, anti-inflammatory and antitumor activity in animals and humans (Neyrinck et al. 2007; Sugiyama et al. 2010; Ji et al. 2012; Xia et al. 2014). Several recent studies addressed the application of ß-glucan from the microalga P. tricornutum. This ß-glucan showed promising cholesterol-lowering properties in zebrafish. The effect of the ß-glucan was similar to Simvastin, a drug used to treat high cholesterol levels (Gora et al. 2022). Reis et al. (2021) showed that ß-glucan from P. tricornutum promotes the health in juvenile fish, allowing for possible application in aqua-feed. Furthermore, feeding trials with ß-glucan-rich P. tricornutum biomass in mice showed gut-related benefits. For example, an increase in short-chain fatty acids was observed in this study (Stiefvatter et al. 2022b). Another recent study with humans described further potentially beneficial effects for healthy aging (Stiefvatter et al. 2022a). These results make algae-derived ß-glucans interesting for human nutrition as well.
Algae-derived ß-glucans can also be used in agriculture. They can act as elicitors to trigger defence mechanisms in vascular plants when they come into contact with their leaves (Kobayashi et al. 1993; Inui et al. 1997; Klarzynski et al. 2000; Aziz et al. 2003; Neyrinck et al. 2007; Sugiyama et al. 2010; Ji et al. 2012; Xia et al. 2014; Wanke et al. 2020). A product with ß-glucan derived from macroalgae is already on the market (Vacciplant™, Stähler). Vascular plants react to a contact with ß-glucans by activating various defence systems against pathogenic fungi such as Plasmopara viticola. Application of ß-glucans may lead to fewer infection events with pathogenic fungi. In grapevine plants treated with ß-glucans, up to 75% fewer infection events with P. viticola were reported compared to the untreated control (Aziz et al. 2003). Other publications report a similar effect for various vascular plants and pathogenic fungi (Kobayashi et al. 1993; Inui et al. 1997; Klarzynski et al. 2000; Wanke et al. 2020).
Besides ß-glucans, microalgae contain further valuable bioactive compounds, such as eicosapentaenoic acid (EPA) and fucoxanthin (FX). All microalgae species tested here contained EPA, while FX is only produced by the two diatoms tested (P. tricornutum and C. fusiformis). EPA is an important omega-3 fatty acid for human nutrition and is already used as a dietary supplement (Ritter et al. 2013). It shows positive effects in the prevention of cardiovascular disease and hypertension (Kang and Leaf 1996; Prisco et al. 1998; Connor 2000; Frenoux et al. 2001; Narayan et al. 2006). In addition, EPA shows antioxidant and anti-inflammatory effects in humans and animals (Kim and Chung 2007; Calder 2010). FX has been reported to exhibit anti-oxidative, anti-inflammatory and weight-reducing properties, as well as activity against non-alcoholic fatty liver disease (NAFLD) (Kotake-Nara et al. 2001; Hosokawa et al. 2004; Maeda et al. 2005, 2006; Sachindra et al. 2007; Heo et al. 2012; Fung et al. 2013; Neumann et al. 2018; Gille et al. 2019). A FX-based product against NAFLD is already available in the United States (Fucovital™, Algatech). Co-products such as FX and EPA, are also interesting for a process focusing on ß-glucan production, as it is possible to extract different compounds from the same biomass through cascaded extraction. This can improve the economical prospect of a production process (Derwenskus et al. 2020b). Therefore, EPA and FX should also be considered to complete the picture for future valorisation in a bio-refinery process.
Although ß-glucans are microalgae-based products with interesting possible applications, there is little information about their production in photobioreactors. Previously published publications on the production of compounds with diatoms tend to focus on pigments (like FX) or fatty acids (like EPA) rather than ß-glucans (Yang et al. 2020). Furthermore, publications that include ß-glucans focus on only one algae species and mostly on better-known algae species such as P. tricornutum (Gao et al. 2017; Frick et al. 2023). Consequently, there is a lack of data regarding the suitability of different microalgae species for the ß-glucan production in scalable commercial photobioreactors. Therefore, we examined and compared the ß-glucan production of the three different algae species P. tricornutum, M. subterranea and C. fusiformis, grown in flat panel airlift reactors (FPA) during nitrogen depletion (N-depletion). Besides ß-glucan, fatty acid accumulation was also analysed. Here, we focused especially on fatty acids which are used as carbon and energy storage by microalgae (here: C16:0, C16:1 and C18:1). In addition, the production of EPA and FX was also analysed, as both are valuable bioactive compounds that can be extracted from the same biomass (Derwenskus et al. 2020b). The algae species selected were a marine species (C. fusiformis), a brackish species (P. tricornutum) and a freshwater species (M. subterranea). In addition to the two diatoms (P. tricornutum and C. fusiformis), the eustigmatophyte M. subterranea was selected for its promising potential to produce EPA and fatty acids (Lu et al. 2002). Furthermore, M. subterranea showed carbohydrate accumulation of up to 300 mg g−1 at steady state in bubble column reactors (Guil-Guerrero and Rebolloso-Fuentes 2008).
Materials and methods
Algae species
Phaeodactylum tricornutum SAG 1090-1b was acquired from the Department of Experimental Phycology and Culture Collection of Algae (EPSAG) of the Georg-August University in Göttingen, Germany. Monodopsis subterranea SAG 848–1 was acquired from the Department of Experimental Phycology and Culture Collection of Algae (EPSAG) of the Georg-August University in Göttingen, Germany. Cylindrotheca fusiformis was acquired from the Australian National Algae Culture Collection (CSIRO).
Culture medium
As brackish cultivation medium for P. tricornutum modified Mann & Myers medium was used, with 10 g L−1 NaCl, 2.4 g L−1 MgSO4.7H2O, 0.6 g L−1 CaCl2.2H2O and 20 ml L−1 trace element solution (Mann and Myers 1968). The trace element solution was prepared as described in the original recipe. Cylindrotheca fusiformis was cultivated under seawater conditions in modified f/2-medium, with 32 g L−1 seawater extract (Tropic Marin, Dr. Biener GmbH), 20 mg L−1 MgSO4.7H2O, 10.6 mg L−1 SiO3 and 2 mL L−1 trace element solution (Guillard and Ryther 1962). Here, the trace element solution was also prepared as described in the original recipe. Monodopsis subterranea was cultivated under freshwater conditions on modified OHM medium, with 493 mg L−1 MgSO4.7H2O, 222 mg L−1 CaCl2.2H2O, 5.2 mg L−1 Fe(III)Citrate.5H2O, 0.02 mg L−1 CoCl2.6H2O, 0.024 mg L−1 CuSO4.5 H2O, 1.98 mg L−1 MnCl2.4H2O, 0.24 mg L−1 Na2MoO3.2H2O (Fábregas et al. 2000). In all media, nitrogen and phosphorous were added separately. A phosphate stock solution was used (50 g L−1) as phosphorous source. The phosphate stock solution was prepared from 45.35 g L−1 K2HPO4 and 35.8 g L−1 KH2PO4. Phosphate concentration in the culture medium ranged from 20 to 200 mg L−1 (0.2—2.1 mmol L−1). As a nitrogen source, ammonium was added from a stock solution (35 g L−1). The ammonium stock solution was prepared from 153.4 g L−1 NH4HCO3. In the pre-cultures (see 3.3), ammonium concentration ranged from 30 to 300 mg L−1 (1.7—16.6 mmol L−1). During the N-depletion, no ammonium was supplied and the ammonium concentration dropped to 0 mg L−1. Ammonium as well as phosphate content of the culture medium was analysed daily using flow injection analysis with a photometrical detector.
Precultures
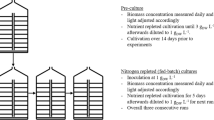
The precultures used for inoculation of the experimental cultures were already cultivated for 14 days under the same cultivation conditions as in the experiments (FPA reactor, pH value, temperature, specific light availability, phosphate concentration). This was done to avoid adaption processes during the experiment. The precultures were regularly diluted so that the biomass concentration (cDW) ranged between 1 and 3 g L−1.
Light regime
Biomass specific light availability (Ispec) was used to describe the light regime. Here, the photon flux density (PFD) on the reactor surface is correlated to the culture volume and the biomass concentration of the culture. Figure S4 shows the PFD applied to the reactor surface. Only light in the PAR region was considered for the calculation. Ispec was described previously by Holdmann et al. (2018). Ispec was calculated according to Eq. 1, using illuminated reactor surface A (0.21 m2), photon flux density PFD on the surface of the reactor, culture volume V and biomass concentration cDW.
Experimental setup and cultivation conditions
For each of the three examined microalgae species, three separate flat panel airlift photobioreactors (culture volume 6 L) were inoculated from the same preculture. These experimental cultures were grown for ten days as batch cultivations. The nitrogen depletion experiments were therefore carried out in biological triplicate for each microalgae species. The duration of N-depletion was chosen based on previous results (Frick et al. 2023). During N-depletion, phosphorous was added throughout the experiment, while no further ammonium was added to the cultures. Subsequently, the ammonium content of the medium dropped to 0 mg L−1. The first day without ammonium in the medium was considered as the start of the N-depletion (Day 0). In all experiments, this was the first day after inoculation, which is why the data is presented up to day 9 of N-depletion.
In all experiments, commercially available flat panel airlift photobioreactors (FPA) with a culture volume of 6 L were used (Subitec GmbH, Germany). The FPA reactor is a variation of a flat plate reactor. An air/CO2 mixture is injected through a silicone membrane at the bottom of the reactor to pneumatically mix the microalgae culture in the reactor. The shape of the reactor has been modified to improve the intermixing of the culture inside. Furthermore, the shape increases the time that the gas bubbles are in the culture medium, which has a positive effect on the gas transfer between gas bubbles and culture medium. The FPA reactors used were equipped with LED panels for artificial illumination (Nichia, NSSL157AT-H3). The LED panels were placed on one side of the reactor at a distance of 2 cm. The LEDs used emitted a light spectrum comparable to sunlight (3000 K, CRI > 90). The FPA reactor was continuously illuminated and the illuminated reactor surface was 0.21 m2. The photon flux density (PFD) on the reactor surface was correlated to the biomass concentration of the culture inside (Ispec). Pre-cultures as well as experimental cultures were cultivated at an Ispec of 5 µmolphotons gDW−1 s−1. Ispec was reset daily to this value by adjusting the impinging PFD on the reactor surface. To control the cultivation conditions, each reactor was equipped with a reactor control unit (Siemens SPS, Germany) that automatically regulated pH value, CO2 content of the gas flow and temperature. PFD on the reactor surface and the addition of phosphate and ammonium had to be adjusted manually. During the experiments, the temperature ranged between 20.0 and 20.5 °C and pH between 7.1 and 7.5 for each species tested. In order to keep the temperature stable, the lower part of the reactors (10 cm) was immersed in a water bath whose temperature was controlled by the reactor control unit. Pressurised air was used for aeration (180 L h−1). To keep the pH value constant, pure CO2 (1—20 L h−1) was automatically added to the airflow.
Analytical procedures
During the experiments, biomass samples were taken daily from each culture using a syringe. The determination of biomass dry weight was carried out with a fresh culture sample. For the analysis of the compounds, the biomass samples were concentrated by centrifugation, washed twice to remove excess medium, frozen and freeze-dried. In preparation for the determination of the beta-glucan content, the fatty acid content, and the fucoxanthin content, cell disruption was carried out using a homogenizer (Precellys24, Bertin technologies, France).
Determination of biomass dry weight
The biomass concentration cDW was determined according to Frick et al. (2023). A sample of 5 mL was put on a pre-dried and pre-weight glass-fibre filter (pore size: 0.2 µm; MN 85/70, Macherey–Nagel GmbH, Germany), placed on a Büchner funnel. After filtering the sample 5 mL of ddH2O was used to remove residual medium from the sample. This step was carried out twice. The filter with the sample then was dried and weighed on an analytical balance. Finally, the biomass concentration cDW was calculated as the difference between the filter loaded with biomass and the empty filter.
Determination of beta-glucan content
The ß-glucan content ωß-glucan (in mg gDW−1) was analysed with an enzymatic test kit (K-EBHLG 08/18, Megazyme, Ireland), which had been used previously for the quantification of ß-glucan from micro- and macroalgae (Danielson et al. 2010; Frick et al. 2023). The test was performed according to the manufacturer’s instructions but scaled down by a factor of 5. In this test, the ß-glucan is enzymatically digested into glucose molecules using a β-1,3-glucanase. Afterwards a glucose oxidase/peroxidase reagent was added, which reacted with the resulting glucose molecules. The resulting colour change was measured photometrically and converted to the initial amount of ß-glucan in the sample (McCleary and Draga 2016). The ß-glucan content of the experimental cultures ωß-glucan was analysed daily in biological triplicates.
Determination of fatty acid content
The total fatty acid content ωTFA, and the content of the specific fatty acids ωEPA, ωC16:0, ωC16:1 and ωC18:1 (all in mg gDW−1) were analysed by gas chromatography (7890A, Agilent, USA) according to the transesterification method described by Lepage and Roy (1984). The content of the different fatty acids of the experimental cultures was analysed daily in biological triplicates.
Determination of fucoxanthin content
The FX content ωFX (in mg gDW−1) was analysed by HPLC (1200 Infinity, Agilent, USA). The method applied was described by Derwenskus et al. (2020a) and is based on the method described by Gille et al. (2015). The FX content ωFX of the experimental cultures was analysed daily in biological triplicates.
Calculations
Volumetric concentration of a compound
The volumetric concentration of a compound X cx (in mg L−1) describes the amount of a compound (here: ß-glucan, FX or fatty acids) per litre culture medium. It was calculated using Eq. 2 with biomass concentration cDW (in g L−1) and the content of the compound X ωX (in mg gDW−1).
Biomass specific beta-glucan productivity
The biomass specific ß-glucan productivity qß-glucan (in mg gDW−1 day−1) describes the amount of ß-glucan which was produced per gram biomass on the previous day. It was calculated with Eq. 3 using the ß-glucan concentration (in mg L−1) at day n (cß-glucan (n)) and at the previous day (cß-glucan (n-1)), as well as the biomass concentration (in g L−1) at the previous day (cDW (n-1)). Furthermore, the observed time window has to be taken into account (here: 1 day).
Statistical analysis
Statistical analysis was performed similar to our latest publication (Frick et al. 2023) using Matlab R2022b (MathWorks, USA). To test for the statistical significance of our results, we employed analysis of variance (ANOVA). The assumptions for ANOVA were checked using the Jarque–Bera test for normality (Matlab function: “jbtest”) and the Bartlett’s test for equal variances (Matlab function: “vartestn”). If these assumptions were not met, Kruskal–Wallis test was used as an alternative to ANOVA to test the statistical significance of our results (Sullivan et al. 2016). ANOVA results are reported using F(df1,df2) and p, where „F” is the F-value, „df1″ and „df2″ are the degrees of freedom and „p” is the p-value. Kruskal–Wallis test results are reported using χ2 (df1, df2) and p. Here, “χ2” is the chi square and “df1” and “df2” are also the degrees of freedom and “p” stands for the p-value. If a significant difference is shown by ANOVA (or Kruskal–Wallis), we used the Tukey post hoc test to determine the significance between the groups. For fucoxanthin analysis, where only two species were compared, we used t-test (Matlab function “ttest”). Results of the t-test are reported using t(df) and p, where „t” is the t-value, „df” is the degree of freedom and „p” is the p-value.
Significant differences (p ≤ 0.05), analysed with ANOVA (or Kruskal–Wallis) and the Tukey post hoc test, are represented with different lowercase letters above the values in tables. If two values are marked with the same letter, this means that there is no significant difference between these two values. The detailed results of all statistical tests performed are presented in Tables S4, S5 and S6.
Results
As described above (see 3.5), the cultures of the three algae species tested were cultivated for nine days under nitrogen depleted conditions. During this period, the increase of the biomass concentration in P. tricornutum and C. fusiformis cultures stopped, whereas biomass concentration in M. subterranea cultures continued to increase thereafter (see Fig. 1). Therefore, the experiments for M. subterranea were continued until biomass increase eventually stopped (day 16). However, to ensure comparability of the results between species, only the first 9 days of N-limitation of all tested algal species were considered in this paper. Additional data on M. subterranea, including protein content, can be found in the appendix (see S3).
Biomass concentration cDW of P. tricornutum, M. subterranea and C. fusiformis during nitrogen depletion. Ispec. was reset daily to 5 µmolphotons gDW−1 s.−1 via adjusting the PFD on the reactor surface (see 3.4). (± SD, n = 3 analysed as biological triplicate). Data for P. tricornutum previously published in Frick et al. (2023)
Biomass increase during nitrogen depletion
Starting with a cDW of 1 g L−1, the biomass concentration cDW increased in all three tested algae species during N-depletion. The maximal biomass concentration differed significantly between C. fusiformis, M. subterranea and P. tricornutum and was highest in M. subterranea cultures (see Table S1). In M. subterranea cultures, the biomass concentration cDW increased up to the last day of the observation period and reached a maximum of 5.18 ± 0.15 g L−1. In P. tricornutum cultures, the highest biomass concentration of 2.70 ± 0.13 g L−1 was measured on day 8 of N-depletion. In C. fusiformis cultures, the biomass concentration did not increase after day 4. The highest biomass concentration for C. fusiformis was measured on day 8 of N-depletion with 1.49 ± 0.31 g L−1.
Accumulation of beta-glucan during nitrogen depletion
The ß-glucan content ωß-glucan increased in all three species during N-depletion. In P. tricornutum and C. fusiformis cultures the ß-glucan content increased fastest in the first few days of N-depletion and did not increase further towards the end of the experiments. The maximal ωß-glucan differed significantly between the species tested (see Table S1). Even though ωß-glucan did not increase in P. tricornutum cultures after day 4, P. tricornutum cultures reached the highest ωß-glucan of all three species tested with 317 ± 9 mg gDW−1 (see Fig. 2 and Table S1). Cylindrotheca fusiformis cultures started with the highest ωß-glucan, but showed the lowest maximal ωß-glucan (129 ± 13 mg gDW−1). After day 4, there was no more increase of ωß-glucan in C. fusiformis cultures. However, in M. subterranea cultures, ωß-glucan increased until the end of the experiment and reached a maximal value of 188 ± 9 mg gDW−1.
ß-glucan content ωß-glucan and volumetric ß-glucan concentration cß-glucan of P. tricornutum, M. subterranea and C. fusiformis during nitrogen depletion. Ispec. was reset daily to 5 µmolphotons gDW−1 s.−1 via adjusting the PFD on the reactor surface (see 3.4). (± SD, n = 3 analysed as biological triplicate see 3.5). Data for ωß-glucan of P. tricornutum previously published in Frick et al. (2023)
With 195 ± 55 mg L−1 C. fusiformis cultures reached a significantly lower maximal volumetric ß-glucan concentration cß-glucan compared to P. tricornutum and M. subterranea cultures. Phaeodactylum tricornutum cultures reached their maximum cß-glucan of 840 ± 66 mg L−1 on day 8 of N-depletion. Whereas in M. subterranea cultures, increasing ß-glucan content and especially increasing biomass concentration led to an increase of cß-glucan until the last day of the experiment (day 9). Subsequently, M. subterranea cultures showed the highest cß-glucan of the three species tested with 975 ± 76 mg L−1.
Accumulation of fatty acids during nitrogen depletion
The accumulation of fatty acids during N-depletion differed between the three species tested with regard to the maximal value and the time course. Total fatty acid (TFA) content ωTFA increased in all three species during N-depletion. Compared to the other two species, M. subterranea cultures showed a significantly higher ωTFA with 299 ± 9 mg gDW−1 on day 9 on N-depletion (see Table 1 and Table S1). In all three species, mostly C16:0 and C16:1 fatty acids were accumulated, as shown by the increasing content of C16:0 ωC16:0 and of C16:1 ωC16:1 (see Table 1). In addition, M. subterranea accumulated C18:1. The EPA content ωEPA remained almost unchanged during N-depletion in C. fusiformis and P. tricornutum but increased in M. subterranea. Overall, M. subterranea exhibited a significantly higher ωEPA with up to 57 ± 2 mg gDW−1 compared to the other two species (see Table 1 and Table S1).
Fucoxanthin under nitrogen depletion
The fucoxanthin content ωFX decreased during N-depletion in P. tricornutum and C. fusiformis cultures. The volumetric fucoxanthin concentration cFX decreased as well in P. tricornutum cultures. Whereas in C. fusiformis cultures, cFX did not change in the progress of N-depletion (see Fig. 3). As a eustigmatophyte, M. subterranea did not produce FX.
Fucoxanthin content ωFX and volumetric fucoxanthin concentration cFX of P. tricornutum and C. fusiformis during nitrogen depletion. As a eustigmatophyte, M. subterranea did not contain any FX. Ispec. was reset daily to 5 µmolphotons gDW−1 s.−1 via adjusting the PFD on the reactor surface (see 3.4). (± SD, n = 3 analysed as biological triplicate). Data for ωFX of P. tricornutum previously published in Frick et al. (2023)
Discussion
The production of any compound always depends on the biomass increase (biomass productivity). Biomass productivity is lower during nutrient depleted cultivation conditions compared to nutrient repleted conditions (Frick et al. 2023). Therefore, N-depletion is mainly favourable for products that accumulate during N-depletion, such as storage compounds like ß-glucans. In our experiments, biomass increased in all three species tested during N-depletion. However, biomass increase in M. subterranea was higher and lasted for a longer period of time compared to the other two species. This had a positive effect on the compounds produced during the process (ß-glucan and fatty acids).
Production of ß-glucan and fatty acids
The accumulation of carbohydrates and fatty acids used as energy storage are closely connected to each other (Siaut et al. 2011; Recht et al. 2012). Microalgae use both carbohydrates and fatty acids as energy storage. It is also reported that carbohydrates such as ß-glucans are metabolised in favour of fatty acids after a longer period of nutrient depletion (Li et al. 2011; Gao et al. 2017). Therefore, the accumulation of ß-glucans and fatty acids during N-depletion should be regarded in context.
For a ß-glucan production process it would be beneficial if the production strain would start accumulating ß-glucan before accumulating fatty acids. In the best case, there would be a considerable amount of time between the starting points. That way, more energy is stored in the form of ß-glucan instead of fatty acids. P. tricornutum cultures started to accumulate ß-glucan prior to fatty acids used as energy storage (C16:0, C16:1). It has been previously described that P. tricornutum accumulates ß-glucan as energy storage prior to the accumulation of fatty acids (Gao et al. 2017). In contrast to P. tricornutum, M. subterranea started the accumulation of ß-glucan and fatty acids simultaneously. Unfortunately, there is no publication to date describing the response of M. subterranea to N-depletion in detail. However, Recht et al. (2012) examined the response of the related eustigmatophyte Nannochloropsis sp. to N-depletion. They reported that Nannochloropsis sp. showed a stable total carbohydrate content during nitrogen depletion. While they did not observe an accumulation of carbohydrates during nitrogen depletion, Nannochloropsis sp. accumulated fatty acids up to 500 mg g−1 (Recht et al. 2012). Cylindrotheca fusiformis accumulated fewer energy storage molecules in response to N-depletion compared to the other two species. Nevertheless, our results for C. fusiformis suggest that the ß-glucan accumulation started before the accumulation of fatty acids. For C. fusiformis there are also no published data describing its response in the progress of nutrient depletion in detail.
The three species showed differences regarding the ratio of ß-glucan and fatty acids used for energy storage (C16:0, C16:1, C18:1). There are microalgae species that prefer fatty acid as energy storage, such as Chlorella vulgaris, and other species favour glucans (starch or ß-glucans) like Chlorella sorokiniana (Mujtaba et al. 2012; Gifuni et al. 2018). For a ß-glucan production process, it would be beneficial if the production strain would favour ß-glucan over fatty acids as energy storage. In this way, more resources (energy and carbon) would be directed to the accumulation of ß-glucan. However, in our experiments, C. fusiformis accumulated more fatty acids than ß-glucan, which is in line with data from a previous publication which suggests that C. fusiformis favours the accumulation of fatty acids over ß-glucan (Schulze et al. 2016). In P. tricornutum cultures, the ß-glucan content was higher than the total fatty acid content in our experiments throughout the N-depletion period. This is in contrast to previous publications (Gao et al. 2017). However, the comparison with other publications is difficult due to differences in the culture conditions, for example light intensity, culture vessel and temperature. In addition, we reported in a previous publication, that the ratio between energy storage molecules differs between different strains of P. tricornutum (Frick et al. 2023). Monodopsis subterranea showed an equal distribution of energy storage molecules in our experiments, which shifted in favour of fatty acids over time. Although there are no published data on this ratio for M. subterranea so far, Recht et al. (2012) reported that the related eustigmatophyte Nannochloropsis sp. accumulated fatty acids rather than carbohydrates during N-depletion. They observed a carbohydrate content of around 200 mg g−1 and a fatty acids content of up to 500 mg g−1 after five days of N-depletion (Recht et al. 2012). This indicates to a species-specific adaption to unfavourable environmental conditions such as N-depletion and emphasises the careful selection of a production strain. However, compared to other publications, the total fatty acid content we found in M. subterranea was rather low (Khozin-Goldberg and Cohen 2006; Hu et al. 2019). This would influence the ratio of ß-glucan to fatty acids in favour of ß-glucan, which would be beneficial for a ß-glucan production process.
The three tested algae species started the formation of energy storage molecules at different times after ammonium was depleted from the medium. It would be advantageous for a ß-glucan production process if the production strain would start the accumulation of ß-glucan shortly after the ammonium in the medium was depleted. In this case, the depletion phase would be shorter. Monodopsis subterranea showed a delayed response to N-depletion regarding the formation of energy storage molecules. The ß-glucan content as well as the content of fatty acids used for energy storage (C:16:0, C16:1, C18:1) started to increase on the third day of N-depletion, whereas the ß-glucan content of P. tricornutum started to increase on the first day. Due to the overall small increase of ß-glucan in C. fusiformis, the beginning of the reaction in this species could not be clearly determined. There are also no published data on this topic so far. The delayed response of M. subterranea to N-depletion indicates that it either has a larger nitrogen storage pool or is able to redistribute its nitrogen resources. In addition to the delayed response, M. subterranea was capable of maintaining biomass formation for a longer period of time (16 days) without ammonium in the culture medium (see Table S2), compared to the other two species (see Fig. 1 and Table S3). This also indicates that M. subterranea is capable to redistribute its nitrogen resources to compensate for the lack of nitrogen in the medium. Furthermore, M. subterranea produces additional ß-glucan far longer compared to the other two species tested.
Overall, our experiments indicate that P. tricornutum and M. subterranea are suitable for ß-glucan production. As described above, P. tricornutum accumulated more ß-glucan than fatty acids and started the accumulation of ß-glucan prior to the accumulation of fatty acids. Accumulation of ß-glucan also began shortly after nitrogen was depleted from the medium and P. tricornutum reached its maximal volumetric ß-glucan concentration earlier compared to M. subterranea, which might be beneficial regarding a possible ß-glucan production process due to a higher space–time yield. In addition, P. tricornutum showed the highest ß-glucan content (see Fig. 2), which is beneficial for possible downstream processing including extraction. Although biomass specific ß-glucan productivity qß-glucan was also higher in P. tricornutum cultures compared to M. subterranea cultures in the beginning of N-depletion (see Figure S3), the maximal volumetric ß-glucan concentration was higher in M. subterranea cultures (see Fig. 2). Taking into account the extended N-depletion time during which M. subterranea produced additional ß-glucan, the difference in the maximal volumetric ß-glucan concentration becomes even greater (see Tables S1 and S3). After 16 days of N-depletion, M. subterranea showed nearly twice the volumetric ß-glucan concentration compared to P. tricornutum. Thus, even considering all the advantages of P. tricornutum listed above, M. subterranea seems to be equally well suited for ß-glucan production. When choosing between these two algae species for ß-glucan production, it might ultimately come down to P. tricornutum producing a soluble ß-glucan (chrysolaminarin) and M. subterranea producing an insoluble ß-glucan (paramylon). C. fusiformis, on the other hand, seems to be less suitable for a ß-glucan production process in the tested setup.
However, the results regarding the ß-glucan production only apply to the tested experimental setup. Cultivation conditions have a great impact on the composition of microalgae biomass. Therefore, light regime, culture density and depleted nutrients might have an impact on the accumulation of ß-glucan as well. Furthermore, it is reported that the cultivation system also has an impact on the performance of the algae (Derwenskus 2020). Flat panel airlift reactors have a good light distribution but impose other requirements on the algae, such as high sheer forces, which can affect the performance of the cultivated algae species (Bergmann 2018; Wang and Lan 2018).
Production of other valuable compounds
The focus of this paper was on the production of ß-glucans. However, to increase the economic feasibility of a potential production process of microalgae compounds, a cascaded extraction as proposed by Derwenskus et al. (2020b) can be beneficial. Therefore, other value-adding components such as FX and fatty acids (especially EPA) have to be considered. FX has been reported to be the major contributor to the biomass value of P. tricornutum due to its high price (Derwenskus et al. 2020b). In a cascaded extraction, the ß-glucan can be separated from the lipophilic compounds (FX, EPA, other fatty acids) by a separation step after cell disruption. To further increase its value, FX can be purified and separated from other lipophilic compounds like EPA. However, this may require an elaborate process such as the chromatography method described by Xiao et al. (2012).
Fucoxanthin
Of the three tested species, FX is only found in the two diatoms, P. tricornutum and C. fusiformis. The FX content decreased in both diatom species during N-depletion. This is consistent with previous publications (Gao et al. 2017). Alipanah et al. (2015) examined the response of P. tricornutum to nutrient depletion at the genetic level and reported that genes associated with FX formation were downregulated during N-depletion. However, although N-depletion is unfavourable for FX production, there is still FX remaining in the biomass to be extracted, even if a short depletion phase would be favourable (see Fig. 3).
EPA
The EPA content of P. tricornutum and M. subterranea increased at the beginning of the N-depletion phase (see Table 1). This is unusual, as EPA is not part of the energy storage pool in the cell. Moreover, other publications reported that EPA content decreased during N-depletion (Khozin-Goldberg and Cohen 2006; Hu et al. 2019). Furthermore, the EPA content of M. subterranea was low at the beginning of the experiments compared to previous publications (Hu et al. 2019). This indicates that the increase in EPA content in our experiments is rather a consequence of the low content at the beginning of the experiment and not due to N-depletion. Nevertheless, our results show that a rather high EPA content (over 45 mgDW g−1) can be achieved during N-depletion (see Table 1). This makes EPA a promising candidate for a cascaded extraction process, especially when using M. subterranea, as EPA content in M. subterranea cultures was still above 45 mgDW g−1 even after 16 days of N-depletion.
During N-depletion, all three tested microalgae species accumulated fatty acids for energy storage (C16:0, C16:1 and C18:1), which could also be extracted after N-depletion (see Table 1). For a cascaded extraction process, M. subterranea and P. tricornutum could be used. The decision depends on the aim of the process. M. subterranea produced more EPA, whereas P. tricornutum additionally produces FX. The high EPA content of M. subterranea makes the biomass also interesting for various applications, such as aqua-feed. Here, the insolubility of its ß-glucan would also be beneficial, as it would not dissolve in the water before being eaten. However, for this application, no extraction would be necessary, only a cell disruption to ensure bioavailability would be needed.
Conclusion
Of the tested algae species, P. tricornutum and M. subterranea are best suited for ß-glucan production. Phaeodactylum tricornutum showed the highest ß-glucan content and reached it in a shorter time compared to M. subterranea. Monodopsis subterranea showed a delayed response to N-depletion, but reached a higher maximal volumetric ß-glucan concentration compared to P. tricornutum. Furthermore, in contrast to P. tricornutum, M. subterranea continued to produce ß-glucan throughout the whole experiment and beyond. In comparison to the other two species tested, C. fusiformis showed a lower accumulation of energy storage molecules (ß-glucan and fatty acids) in response to N-depletion. For a production process, other valuable compounds should be considered besides ß-glucan. EPA content is still rather high during N-depletion, especially in M. subterranea and although FX content of P. tricornutum and C. fusiformis decreased during N-depletion, it could still be extracted in a cascaded extraction to increase value for these biomasses.
Data availability
All data generated or analysed during this study are included in this published article. The data and materials used in this paper comply with field standards, all cultivations were conducted in biological triplicate.
References
Adamakis I-D, Lazaridis PA, Terzopoulou E, Torofias S, Valari M, Kalaitzi P, Rousonikolos V, Gkoutzikostas D, Zouboulis A, Zalidis G, Triantafyllidis KS (2018) Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Env Science Poll Res Int 25:23018–23032
Alipanah L, Rohloff J, Winge P, Bones AM, Brembu T (2015) Whole-cell response to nitrogen deprivation in the diatom Phaeodactylum tricornutum. J Exp Bot 66:6281–6296
Aziz A, Poinssot B, Daire X, Adrian M, Bézier A, Lambert B, Joubert J-M, Pugin A (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant-Microbe Interact 16:1118–1128
Bergmann P (2018) Operating strategy to reduce the energy consumption of flat-panel airlift photobioreactors with respect to mixing of Thermosynechococcus elongatus suspension cultures light-specific adaptation of the superficial gas velocity. Dissertation, University of Hohenheim, Germany
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2:355–374
Ciecierska A, Drywień ME, Hamulka J, Sadkowski T (2019) Nutraceutical functions of beta-glucans in human nutrition. Roczniki Państwowego Zakładu Higieny 70:315–324
Connor WE (2000) Importance of n-3 fatty acids in health and disease. Am J Clin Nutr 71:171S-5S
Danielson ME, Dauth R, Elmasry NA, Langeslay RR, Magee AS, Will PM (2010) Enzymatic method to measure β-1,3-β-1,6-glucan content in extracts and formulated products (GEM assay). J Ag Food Chem 58:10305–10308
Derwenskus F (2020) Entwicklung und Bewertung eines Verfahrens zur Herstellung von Fucoxanthin und Eicosapentaensäure mit Phaeodactylum tricornutum. Dissertation, University of Stuttgart, Germany.
Derwenskus F, Schäfer B, Müller J, Frick K, Gille A, Briviba K, Schmid‐Staiger U, Hirth T (2020a) Coproduction of EPA and fucoxanthin with P. tricornutum – A promising approach for up‐ and downstream processing. Chemie Ingenieur Technik 92:1780–1789
Derwenskus F, Weickert S, Lewandowski I, Schmid-Staiger U, Hirth T (2020b) Economic evaluation of up- and downstream scenarios for the co-production of fucoxanthin and eicosapentaenoic acid with P. tricornutum using flat-panel airlift photobioreactors with artificial light. Algal Res 51:102078
Eliáš M, Amaral R, Fawley KP, Fawley MW, Němcová Y, Neustupa J, Přibyl P, Santos LMA, Ševčíková T (2017) Eustigmatophyceae. In: Archibald JM, Simpson AGB, Slamovits CH (eds) Handbook of the protists, 2nd edn. Springer, Cham, pp 367–406
Espinoza-Gallardo D, Contreras-Porcia L, Ehrenfeld N (2017) ß-glucanos, su producción y propiedades en microalgas con énfasis en el género Nannochloropsis (Ochrophyta, Eustigmatales). Rev Biol Mar Oceanogr 52:33–49
Fábregas J, Domínguez A, Regueiro M, Maseda A, Otero A (2000) Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Appl Microbiol Biotechnol 53:530–535
Frenoux JM, Prost ED, Belleville JL, Prost JL (2001) A polyunsaturated fatty acid diet lowers blood pressure and improves antioxidant status in spontaneously hypertensive rats. J Nutr 131:39–45
Frick K, Yeh Y-C, Schmid-Staiger U, Tovar GEM (2023) Comparing three different Phaeodactylum tricornutum strains for the production of chrysolaminarin in flat panel airlift photobioreactors. J Appl Phycol 35:11–24
Fung A, Hamid N, Lu J (2013) Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem 136:1055–1062
Gao B, Chen A, Zhang W, Li A, Zhang C (2017) Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J Ocean Univ China 16:916–924
Gifuni I, Olivieri G, Pollio A, Marzocchella A (2018) Identification of an industrial microalgal strain for starch production in biorefinery context: The effect of nitrogen and carbon concentration on starch accumulation. New Biotech 41:46–54
Gille A, Stojnic B, Derwenskus F, Trautmann A, Schmid-Staiger U, Posten C, Briviba K, Palou A, Bonet ML, Ribot J (2019) A lipophilic fucoxanthin-rich Phaeodactylum tricornutum extract ameliorates effects of diet-induced obesity in C57BL/6J mice. Nutrients 11:796
Gille A, Trautmann A, Posten C, Briviba K (2015) Bioaccessibility of carotenoids from Chlorella vulgaris and Chlamydomonas reinhardtii. Int J Food Sci Nutr 67:507–513
Gora AH, Rehman S, Kiron V, Dias J, Fernandes JMO, Olsvik PA, Siriyappagouder P, Vatsos I, Schmid-Staiger U, Frick K, Cardoso M (2022) Management of hypercholesterolemia through dietary ß-glucans–Insights from a zebrafish model. Front Nutr 8:1590
Gruber A, Kroth PG (2017) Intracellular metabolic pathway distribution in diatoms and tools for genome-enabled experimental diatom research. Phil Trans R Soc Lond B 372:20160402
Guil-Guerrero JL, Rebolloso-Fuentes MM (2008) Nutrient composition of Chlorella spp. and Monodus subterraneus cultured in a bubble column bioreactor. Food Biotech 22:218–233
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Heo S-J, Yoon W-J, Kim K-N, Oh C, Choi Y-U, Yoon K-T, Kang D-H, Qian Z-J, Choi I-W, Jung W-K (2012) Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chemical Toxicol 50:3336–3342
Holdmann C, Schmid-Staiger U, Hornstein H, Hirth T (2018) Keeping the light energy constant — Cultivation of Chlorella sorokiniana at different specific light availabilities and different photoperiods. Algal Res 29:61–70
Hosokawa M, Kudo M, Maeda H, Kohno H, Tanaka T, Miyashita K (2004) Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta 1675:113–119
Hu H, Li J-Y, Pan X-R, Zhang F, Ma L-L, Wang H-J, Zeng RJ (2019) Different DHA or EPA production responses to nutrient stress in the marine microalga Tisochrysis lutea and the freshwater microalga Monodus subterraneus. Sci Total Environ 656:140–149
Inui H, Yamaguci Y, Hirano S (1997) Elicitor actions of N-acetylchitooligosaccharides and laminarioligosaccharides for chitinase and L-phenylalanine ammonia-lyase induction in rice suspension culture. Biosci Biotech Biochem 61:975–978
Ji YB, Ji CF, Zhang H (2012) Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules 17:9947–9960
Kang JX, Leaf A (1996) Antiarrhythmic effects of polyunsaturated fatty acids. Recent studies. Circulation 94:1774–1780
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Kim YJ, Chung HY (2007) Antioxidative and anti-inflammatory actions of docosahexaenoic acid and eicosapentaenoic acid in renal epithelial cells and macrophages. J Med Food 10:225–231
Kiss JZ, Vasconcelos AC, Triemer RE (1988) The intramembranous particle profile of the paramylon membrane during paramylon synthesis in Euglena (Euglenophyceae). J Phycol 24:152–157
Klarzynski O, Plesse B, Joubert J-M, Yvin J-C, Kopp M, Kloareg B, Fritig B (2000) Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol 124:1027–1038
Kobayashi A, Tai A, Kanzaki H, Kawazu K (1993) Elicitor-active oligosaccharides from algal laminaran stimulate the production of antifungal compounds in alfalfa. Z Naturforsch C 48:575–579
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) Carotenoids affect proliferation of human prostate cancer cells. J Nutr 131:3303–3306
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129
Lu C, Acién Fernández FG, Cañizares Guerrero E, Hall DO, Molina Grima E (2002) Overall assessment of Monodus subterraneus cultivation and EPA production in outdoor helical and bubble column reactors. J Appl Phycol 14:331–342
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K (2005) Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun 332:392–397
Maeda H, Hosokawa M, Sashima T, Takahashi N, Kawada T, Miyashita K (2006) Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int J Molec Med 18:147–152
Mann JE, Myers J (1968) On pigments, growth and photosynthesis of Phaeodactylum tricornutum. J Phycol 4:349–355
McCleary BV, Draga A (2016) Measurement of beta-glucan in mushrooms and mycelial products. J AOAC Int 99:364–373
Mujtaba G, Choi W, Lee C-G, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283
Myklestad SM (1989) Production, chemical structure, metabolism, and biological function of the (1→3)-linked, β3-D-glucans in diatoms. Biol Oceanogr 6:313–326
Narayan B, Miyashita K, Hosakawa M (2006) Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Food Rev Int 22:291–307
Neumann U, Louis S, Gille A, Derwenskus F, Schmid-Staiger U, Briviba K, Bischoff SC (2018) Anti-inflammatory effects of Phaeodactylum tricornutum extracts on human blood mononuclear cells and murine macrophages. J Appl Phycol 30:2837–2846
Neyrinck AM, Mouson A, Delzenne NM (2007) Dietary supplementation with laminarin, a fermentable marine beta (1–3) glucan, protects against hepatotoxicity induced by LPS in rat by modulating immune response in the hepatic tissue. Intl Immunopharmacol 7:1497–1506
Prisco D, Paniccia R, Bandinelli B, Filippini M, Francalanci I, Giusti B, Giurlani L, Gensini GF, Abbate R, Serneri GGN (1998) Effect of medium-term supplementation with a moderate dose of n-3 polyunsaturated fatty acids on blood pressure in mild hypertensive patients. Thrombosis Res 91:105–112
Recht L, Zarka A, Boussiba S (2012) Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl Microbiol Biotechnol 94:1495–1503
Reis B, Gonçalves AT, Santos P, Sardinha M, Conceição LEC, Serradeiro R, Pérez-Sánchez J, Calduch-Giner J, Schmid-Staiger U, Frick K, Dias J, Costas B (2021) Immune status and hepatic antioxidant capacity of gilthead seabream Sparus aurata juveniles fed yeast and microalga derived β-glucans. Mar Drugs 19:653
Ritter JCS, Budge SM, Jovica F (2013) Quality analysis of commercial fish oil preparations. J Sci Food Agricult 93:1935–1939
Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M, Miyashita K (2007) Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Ag Food Chem 55:8516–8522
Schulze C, Wetzel M, Reinhardt J, Schmidt M, Felten L, Mundt S (2016) Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J Appl Phycol 28:2719–2725
Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotech 11:7
Stiefvatter L, Frick K, Lehnert K, Vetter W, Montoya-Arroyo A, Frank J, Schmid-Staiger U, Bischoff SC (2022a) Potentially beneficial effects on healthy aging by supplementation of the EPA-rich microalgae Phaeodactylum tricornutum or its supernatant-A randomized controlled pilot trial in elderly individuals. Mar Drugs 20:716
Stiefvatter L, Neumann U, Rings A, Frick K, Schmid-Staiger U, Bischoff SC (2022b) The microalgae Phaeodactylum tricornutum is well suited as a food with positive effects on the intestinal microbiota and the generation of SCFA: Results from a pre-clinical study. Nutrients 14:2504
Sugiyama A, Hata S, Suzuki K, Yoshida E, Nakano R, Mitra S, Arashida R, Asayama Y, Yabuta Y, Takeuchi T (2010) Oral administration of paramylon, a β-1,3-D-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J Vet Med Sci 72:755–763
Sullivan LM, Weinberg J, Keaney JF (2016) Common statistical pitfalls in basic science research. J Amer Heart Assoc 5:e004142
Vieler A, Wu G, Tsai C-H, Bullard B, Cornish AJ, Harvey C, Reca I-B, Thornburg C, Achawanantakun R, Buehl CJ, Campbell MS, Cavalier D, Childs KL, Clark TJ, Deshpande R, Erickson E, Armenia Ferguson A, Handee W, Kong Q, Li X, Liu B, Lundback S, Peng C, Roston RL, Sanjaya, Simpson JP, TerBush A, Warakanont J, Zäuner S, Farre EM, Hegg EL, Jiang N, Kuo M-H, Lu Y, Niyogi KK, Ohlrogge J, Osteryoung KW, Shachar-Hill Y, Sears BB, Sun Y, Takahashi H, Yandell M, Shiu S-H, Benning C (2012) Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genetics 8:e1003064
Wang C, Lan CQ (2018) Effects of shear stress on microalgae – A review. Biotechnol Adv 36:986–1002
Wanke A, Rovenich H, Schwanke F, Velte S, Becker S, Hehemann J-H, Wawra S, Zuccaro A (2020) Plant species-specific recognition of long and short β-1,3-linked glucans is mediated by different receptor systems. Plant J 102:1142–1156
Xia S, Gao B, Li A, Xiong J, Ao Z, Zhang C (2014) Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar Drugs 12:4883–4897
Xiao X, Si X, Yuan Z, Xu X, Li G (2012) Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J Sep Sci 35:2313–2317
Yang R, Wei D, Xie J (2020) Diatoms as cell factories for high-value products: chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit Rev Biotech 40:993–1009
Yodsuwan N, Sawayama S, Sirisansaneeyakul S (2017) Effect of nitrogen concentration on growth, lipid production and fatty acid profiles of the marine diatom Phaeodactylum tricornutum. Agricult Nat Resour 51:190–197
Acknowledgements
This work was supported by the State Ministry of Baden-Wuerttemberg for Sciences, Research and Arts (Fkz.: 7533-10-5-185A) and the State Ministry of Baden-Wuerttemberg of Food, Rural Affairs and Consumer Protection (Fkz.: BWFE120131 and 54-8214.07-FP20-128/1).
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the State Ministry of Baden-Wuerttemberg for Sciences, Research and Arts (Fkz.: 7533–10-5-185A) and the State Ministry of Baden-Wuerttemberg of Food, Rural Affairs and Consumer Protection (Fkz.: BWFE120131 and 54–8214.07-FP20-128/1).
Author information
Authors and Affiliations
Contributions
KF: Conceptualization, Methodology, Validation, Investigation, Data Curation, Writing—Original Draft, Visualization.
TE: Methodology, Visualization, Writing—Review & Editing.
USS: Conceptualization, Methodology, Resources, Writing—Review & Editing, Supervision, Project administration, Funding acquisition.
YCY: Formal analysis, Data Curation, Writing—Review & Editing.
GEMT: Resources, Writing—Review & Editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frick, K., Ebbing, T., Yeh, YC. et al. Beta-glucan production of Phaeodactylum tricornutum, Monodopsis subterranea and Cylindrotheca fusiformis during nitrogen depletion. J Appl Phycol 35, 2607–2618 (2023). https://doi.org/10.1007/s10811-023-03026-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03026-8