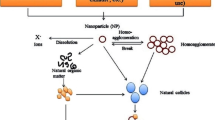
Abstract
There is increasing interest and need to develop a deeper understanding of the nature, fate and behaviour of nanoparticles in the environment. This is driven by the increased use of engineered nanoparticles and the increased pressure to commercialise this growing technology. In this review we discuss the key properties of nanoparticles and their preparation and then discuss how these factors can play a role in determining their fate and behaviour in the natural environment. Key focus of the discussion will relate to the surface chemistry of the nanoparticle, which may interact with a range of molecules naturally present in surface waters and sediments. Understanding these factors is a core goal required for understanding the final fate of nanomaterials and predicting which organisms are likely to be exposed to these materials.













Similar content being viewed by others
References
Baalousha M (2008) Aggregation and disaggregation of iron oxide nanoparticles; influence of particles concentration, pH and natural organic matter. J Nanopart Res (Submitted)
Baalousha M, Alexa N-A, Cieslak E, Lead JR (2008a) Transport mechanisms of carbon nanotubes in the natural aquatic environment. Environ Sci Technol (Submitted)
Baalousha M, Lead JR (2007) Characterization of natural aquatic colloids (<5 nm) by flow-field flow fractionation and atomic force microscopy. Environ Sci Technol 41:1111–1117
Baalousha M, Manciulea A, Cumberland S, Kendall K, Lead JR (2008b) Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter. Environ Toxicol Chem (in press)
Ballesteros E, Gallego M, Valcarcel M (2000) Analytical potential of fullerene as adsorbent for organic and organometallic compounds from aqueous solutions. J Chromatogr A 869:101–110
Biswas P, Wu CY (2005) Nanoparticles and the environment. J Air Waste Manage Assoc 55:708–746
Brant J, Lecaotnet H, Wiessner MR (2005) Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanopart Res 7:533–545
Buffle J (2006) The key role of environmental colloids/nanoparticles for the sustainablility of life. Environ Chem 3:155–158
Buffle J, Wilkinson KJ, Stoll S, Filella M, Zhang J (1998) A generalized description of aquatic colloidal interactions: the three-colloidal component approach. Environ Sci Technol 32:2887–2899
Cai YQ, Cai Y, Mou Sf, Lu Yq (2005) Multi-walled carbon nanotubes as a solid-phase extraction adsorbent for the determination of chlorophenols in environmental water samples. J Chromatogr A 1081:245–247
Chen KL, Elimelech M (2006) Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 22:10994–11001
Chen C, Wang X (2006) Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind Eng Chem Res 45:9144–9149
Chen KL, Mylon SE, Elimelech M (2006) Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ Sci Technol 40:1516–1523
Chen KL, Mylon SE, Elimelech M (2007) Enhanced aggregation of alginate-coated iron oxide (Hematite) nanoparticles in the presence of calcium, strontium, and barium cations. Langmuir 23:5920–5928
Cheng X, Kan AT, Tomson MB (2004) Naphthalene adsorption and desorption from aqueous C60 fullerene. J Chem Eng Dat 49:675–683
Christian P, O’Brien P (2005) A new route to CdS nanorods. Chem Commun 2817–2819
Dhage SR, Pasricha R, Ravi V (2003) Synthesis of ultrafine TiO2 by citrate gel method. Mat Res Bull 38:1623–1628
Ding Q, Liang P, Song F, Xiang A (2006) Separation and preconcentration of silver ion using multiwalled carbon nanotubes as solid phase extraction sorbent. Sep Sci Technol 41:2723–2732
Dios M, Barroso F, Tojo C, Blanco MC, Lopez-Quintela MA (2005) Effects of the reaction rate on the size control of nanoparticles synthesized in microemulsions. Coll Surf: Physiochem Eng Aspects 270–271:83–87
Dupuis AC (2005) The catalyst in the CCVD of carbon nanotubes—a review. Prog Mat Sci 50:929–961
Esquivel EV, Murr LE (2004) A TEM analysis of nanoparticulates in a Polar ice core. Mater Charact 52(1):15–25
Giammar DE, Maus CJ, Xie L (2007) Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles. Environ Eng Sci 24:85–95
Giasuddin ABM, Kanel SR, Choi H (2007) Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ Sci Technol 41:2022–2027
Gotovac S, Hattori Y, Noguchi D, Miyamoto J, Kanamaru M, Utsumi S, Kanoh H, Kaneko K (2006) Phenanthrene adsorption from solution on single wall carbon nanotubes. J Phys Chem B 110:16219–16224
Hague DC, Mayo MJ (1994) Controlling crystallinity during processing of annocrystalline titania. J Am Ceram Soc 77:1957–1960
Helland A, Wick P, Koehler A, Schmid K, Som C (2007) Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ Health Perspec 115:1125–1131
Henglein A, Giersig M (1999) Formation of colloidal silver nanoparticles: capping action of citrate. J Phys Chem B 103:9533–9539
Huang PM, Wang MK, Chiu CY (2005) Soil mineral-organic matter-microbe interactions: impacts on biogeochemical processes and biodiversity in soils. Pedobiologia 49:609–635
Hunter KA, Liss PS (1982) Organic matter and surface charge of suspended particles in estuarine waters. Limnol Oceanogr 27:322–335
Hyung H, Fortner JD, Hughes JB, Kim JH (2007) Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ Sci Technol 41:179–184
IUPAC (1997) IUPAC compendium of chemical terminology, 2nd edn, compiled by McNaught, AD, Wilkinson A. Blackwell Science, ISBN 0865426848. http://old.iupac.org/publications/compendium/index.html
Iwasaki T (1937) Some nites on sand filtration. J Am Wat Works Assoc 29:1591–1602
Jarvis P, Jefferson B, Gregory J, Parsons SA (2005) A review of floc strength and breakage. Water Res 39:3121–3137
Jekel MR (1986) The stabilization of dispersed mineral particles by adsorption of humic substances. Water Res 20:1543–1554
Johnson CP, Li X, Logan BE (1996) Settling velocities of fractal aggregates. Environ Sci Technol 30:1911–1918
Kreyling WG, Semmler-Behnke M, Möller W (2006) Health implications of nanoparticles. J Nanopart Res 8:543–562
Kukovitsky EF, L’vov SG, Sainov NA (2000) VLS-growth of carbon nanotubes from the vapour. Chem Phys Lett 317:65–70
Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL (2006) A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol 36:189–217
Lead JR, Hamilton-Taylor J, Davison W, Harper M (1999) Trace metal sorption by natural particles and coarse colloids. Geochim Cosmochim Acta 63:1661–1670
Lead JR, Muirhead D, Gibson CT (2005) Characterisation of freshwater natural aquatic colloids by atomic force microscopy (AFM). Environ Sci Technol 39:6930–6936
Lead JR, Wilkinson KJ (2006) Aquatic colloids and nanoparticles: current knowledge and future trends. Environ Chem 3:159–171
Li XY, Logan BE (2001) Permeability of fractal aggregates. Water Res 35:3373–3380
Li YH, Wang S, Wei J, Zhang X, Xu C, Luan Z, Wu D, Wei B (2002) Lead adsorption on carbon nanotubes. Chem Phys Lett 357:263–266
Liang P, Liu Y, Guo L, Zeng J, Lu H (2004) Multiwalled carbon nanotubes as solid-phase extraction adsorbent for the preconcentration of trace metal ions and their determination by inductively coupled plasma atomic emission spectrometry. J Anal Atom Spectrom 19:1489–1492
Liang P, Liu Y, Guo L (2005) Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes. Spectrochim Acta B: Atom Spectroscop 60:125–129
Liang P, Ding Q, Song F (2006) Application of multiwalled carbon nanotubes as solid phase extraction sorbent for preconcentration of trace copper in water samples. J Sep Sci 28:2339–2343
Long RQ, Yang RT (2001) Carbon nanotubes as superior sorbent for dioxin removal. J Am Chem Soc 123:2058–2059
Lu C, Chiu H (2006) Adsorption of zinc(II) from water with purified carbon nanotubes. Chem Eng Sci 61:1138–1145
Lu C, Chung YL, Chang KF (2005) Adsorption of trihalomethanes from water with carbon nanotubes. Water Res 39:1183–1189
Luo X, Killard AJ, Morrin A, Smyth MR (2007) Electrochemical preparation of distinct polyaniline nanostructrues by surface charge control of polystyrene nanoparticle templates. Chem Commun 3207–3209
Lyven B, Hassellov M, Turner DR, Haraldsson C, Andersson K (2003) Competition between iron- and carbon-based colloidal carriers for trace metals in a freshwater assessed using flow field-flow fractionation coupled to ICPMS. Geochim Cosmochim Acta 67:3791–3802
Madden AS, Hochella J, Luxton TP (2006) Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim Cosmochim Acta 70:4095–4104
Malik MA, O’Brien P, Revaprasadu N (2002) A simple route to the synthesis of core/shell nanoparticles of chalcogenides. Chem Mat 14:2004–2010
Mana L, Scher EC, Alivisatos AP (2000) Synthesis of soluble and processable rod-, arrow-, teardor-, and tetrapod-shaped CdSe nanocrystals. J Am Chem Soc 112:12700
Mattigod SV, Fryxell GE, Skaggs R, Parker KE (2006) Functionalized nanoporous ceramic sorbents for removal of mercury and other contaminants. Nano Science and Technology Institute. Technical Proceedings of the 2006 NSTI Nanotechnology Conference and Trade Show, vol 1, pp 355–357
McCarthy JF, McKay LD (2004) Colloid transport in the subsurface: past, present, and future challenges. Vadose Zone J 3:326–337
McCarthy JF, Zachara JM (1989) Subsurface transport of contaminants. Environ Sci Technol 23:496–502
McDowell-Boyer LM, Hunt JR, Sitar N (1986) Particle transport through porous media. Water Resour Res 22:1901–1921
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
NIOSH (2004) What is nanotechnology publication 2004-175. http://www.cdc.gov/niosh/docs/2004-175/pdfs/2004-175.pdf
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Poll 150:5–22
O’Melia CR, Tiller CL (1993) Physiochemical aggregation and deposition in aquatic environments. In: Buffle J, van Leuween HP (eds) Environ part, vol 2. Lewis Publishers, London, pp 353–386
Obare SO, Meyer GJ (2005) Nanostructured materials for environmental remediation of organic contaminants in water. J Environ Sci Health A 39:2549–2582
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles supplemental web sections. Environ Health Perspec 113:823–839
Ouali L, Pefferkorn E (1994a) Fragmentation of colloidal aggregates induced by polymer adsorption. J Coll Interf Sci 168:315–322
Paparazzo E (1992) Evidence of Si-OH species on the surface of aged silica. J Vac Sci Technol 10:2892–2896
Park SK, Kim KD, Kim HT (2002) Preparation of silica nanoparticles: determination of the optimal synthesis conditions for small and uniform particles. Coll Surf 197:7–17
Pefferkorn E (1995) The role of polyelectrolytes in the stabilisation and destabilisation of colloids. Adv Coll Interf Sci 56:33–104
Peng X, Li Y, Luan Z, Di Z, Wang H, Tian B, Jia Z (2003) Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem Phys Lett 376:154–158
Phenrat T, Saleh N, Sirk K, Tilton RD, Lowry GV (2007) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290
Pons M, Garcia ML, Valls O (1991) Influence of stabilizers on particle size and polydispersity of polybutyl- and polyisobutil-cyanoacrylate nanoparticles. Coll Pol Sci 269:855–858
Qiu S, Dong J, Chen G (1999) Preparation of Cu nanoparticles from water-in-oil microemulsions. J Coll Interf Sci 216:230–234
Rajagopalan R, Tien C (1976) Trajectoty analysis of deep-bed filtration with the sphere-in-cell porous media model. Am Inst Chem Ing 22:523–533
Rempel JY, Trout BT, Bawendi MG, Jensen KF (2006) Density functional theory study of ligand binding on CdSe (0001), (0001), and (1120) single crystal relaxed and reconstructed surfaces: implications for nanocrystalline growth. J Phys Chem B 110:18007–18016
Rizzi FR, Stoll S, Senesi N, Buffle J (2004) A transmission electron microscopy study of the fractal properties and aggregation processes of humic acids. Soil Sci 169:765–775
Ruckenstein E, Prieve DC (1973) Rate of deposition of Brownian particles under action of London and double-layer forces. J Chem Soc Far Trans 2 69(10):1522–1536
Rudalevige T, Francis AH, Zand R (1998) Spectroscopic studies of fullerene aggregates. J Phys Chem A 102:9797–9802
Ryan JN, Gschwend PM (1994) Effect of solution chemistry on clay colloid release from an iron-oxide-coated aquifer sand. Environ Sci Technol 28:1717–1726
Sau TK, Pal A, Pal T (2001) Size regime dependent catalysis by gold nanoparticles for the reduction of eosin. J Phys Chem B 105:9266–9272
SCENIHR (2005) Request for a scientific opinion on the appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious nanotechnologies. SCENIHR/002/05
SCENIHR (2007) Opinion on the appropriateness of the risk assessment methodology in accordance with the technical guidance documents for new and existing substances for assessing the risks of nanomaterials. European Commission Heath and Consumer Protection Directorate-General
Shaw DJ (1992) Colloid and surface science, 4th edn. Butterworth-Heinemann Ltd
Shim SE, Lee H, Soonja C (2004) Synthesis of functionalized monodisperse poly(methyl methacryacrylate) nanoparticles by a RAFT agent carrying carboxyl eng group. Macromol 37:5565–5571
Sokolowska Z, Sokolowski S (1999) Influence of humic acid on surface fractal dimension of kaolin: analysis of mercury porosimetry and water vapour adsorption data. Geoderma 88:233–249
Spielmann LA, Cukor PM (1973) Deposition of non-Brownian particles under colloidal forces. J Coll Interface Sci 43:51–61
Stumm W (1992) Chemistry of the solid-water interface. Processes at the mineral-water and particle-water interface in natural waters. Wiley-Interscience, New York
Stumm W, Morgan JJ (1996) Aquatic chemistry. Chemical equilibria and rates in natural waters, 3rd edn. Wiley-Interscience, New York
Sun CH, Li F, Ying Z, Liu C, Cheng HM (2004) Surface fractal dimension of single-walled carbon nanotubes. Phys Rev B 69:033404-1-033404-4
Sun Y, Li X, Zhang W, Wang P (2007) A method for the preparation of stable dispersions of zero-valent iron nanoparticles. Coll Surf A: Physicochem Eng Aspects 31:60–66
The Royal Society & The Royal Academy of Engineering (2004) Nanoscience and nanotechnologies: opportunities and uncertainties. http://www.nanotec.org.uk/finalReport.htm
Tien C, Payatakes AC (1979) Advances in deep bed filtration. Am Inst Chem Eng J 25:737–759
Tipping E, Higgins DC (1982) The effect of adsorbed humic substances on the colloid stability of haematite particles. Coll Surf 5:85–92
Tipping E, Ohnstad M (1984) Colloid stability of iron oxide particles from a freshwater lake. Nature 308:266–268
Trindade T, O’Brien P, Pickett N (2001) Nanocrystalline semiconductors: synthesis, properties, and perspectives. Chem Mater 13:3843–3858
Tsantilis S, Kammler HK, Pratsinis SE (2002) Population balance modeling of flame synthesis of titania nanoparticles. Chem Eng Sci 57:2139–2156
Ung D, Soumare Y, Chakroune N, Viau G, Vaulay M, Richard V, Fievet F (2007) Growth of magnetic nanowires and nanodumbbells in liguid polyol. Chem Mater 19:2084–2094
Wang X, Chen C, Hu W, Ding A, Xu D, Zhou X (2005) Sorption of 243Am(III) to multiwall carbon nanotubes. Environ Sci Technol 39:2856–2860
Wang JX, Jiang DQ, Gu ZY, Yan XP (2006) Multiwalled carbon nanotubes coated fibers for solid-phase microextraction of polybrominated diphenyl ethers in water and milk samples before gas chromatography with electron-capture detection. J Chromatogr A 1137:8–14
Wang X, Li Y (2006) Solution based synthetic strategies for 1D nanostructures. Inorg Chem 45:7522–7534
Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P (2006) Assessing the risks of manufactured nanomaterials. Environ Sci Technol 40:4336–4345
Wiggington NS, Huas KL, Hochella MF (2007) Aquatic environmental nanoparticles. J Environ Monitor 9:1306–1316
Winter JO, Gomez N, Gatzert S, Schmidt CE, Korgel BA (2005) Variation of cadmium sulfide nanoparticle size and photoluminescence intensity with altered aqueous synthesis conditions. Coll Surf A 254:147–157
Wuister SF, Donega CM, Meijerink A (2004) Infuence of thiol capping on the exciton luminescence and decay kinetics of CdTe and CdSe quantum dots. J Phys Chem B 108:17393–17397
Yang K, Xing B (2007) Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ Pollut 145:529–537
Yao K-M (1968) Influence of suspended particle size on the transport aspect of water filtration. Diss Univ of North Carolina, Chapel Hill
Yao K-M, Habbibian MT, O’Melia CR (1971) Water and wastewater filtration: concepts and applications. Environ Sci Technol 5:1105–1112
Yeung AKC, Pelton R (1996) Micromechanics: a new approach to studying the strength and breakup of flocs. J Colloid Interf Sci 184:579–585
Yonezawa T, Kunitake T (1999) Practical preparation of anionic mercapto ligand-stabilized gold nanoparticles and their immobilization. Coll Surf A 149:193–199
Yoo JS (1998) Selective gas-phase oxidation at oxide nanoparticles on microporous materials. Catal Today 41:409–432
Zhang W-S (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Zhou Q, Ding Y, Xiao J (2006) Sensitive determination of thiamethoxam, imidacloprid and acetamiprid in environmental water samples with solid-phase extraction packed with multiwalled carbon nanotubes prior to high-performance liquid chromatography. Anal Bioanal Chem 385:1520–1525
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christian, P., Von der Kammer, F., Baalousha, M. et al. Nanoparticles: structure, properties, preparation and behaviour in environmental media. Ecotoxicology 17, 326–343 (2008). https://doi.org/10.1007/s10646-008-0213-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0213-1