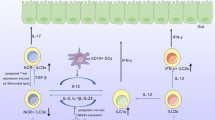
Abstract
Inflammatory bowel disease (IBD), encompassing Crohn’s disease and ulcerative colitis, is a chronic intestinal inflammatory disorder characterized by diffuse accumulation of lymphocytes in the gut mucosa as a consequence of over-expression of endothelial adhesion molecules. The infiltrating lymphocytes have been identified as subsets of T cells, including T helper (Th)1 cells, Th17 cells, and regulatory T cells. The function of these lymphocyte subpopulations in the development of IBD is well-known, since they produce a number of pro-inflammatory cytokines, such as interferon-γ and interleukin-17A, which in turn activate mucosal proteases, thus leading to the development of intestinal lesions, i.e., ulcers, fistulas, abscesses, and strictures. However, the immune mechanisms underlying IBD are not yet fully understood, and knowledge about the function of newly discovered lymphocytes, including Th9 cells, innate lymphoid cells, mucosal-associated invariant T cells, and natural killer T cells, might add new pieces to the complex puzzle of IBD pathogenesis. This review summarizes the recent advances in the understanding of the role of mucosal lymphocytes in chronic intestinal inflammation and deals with the therapeutic potential of lymphocyte-targeting drugs in IBD patients.


Similar content being viewed by others
Abbreviations
- Breg:
-
Regulatory B cell
- CD:
-
Crohn’s disease
- IBD:
-
Inflammatory bowel disease
- IFN:
-
Interferon
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- ILC:
-
Innate lymphoid cell
- IL-13R:
-
Interleukin-13 receptor
- JAK:
-
Janus kinase
- MAIT:
-
Mucosal-associated invariant T
- NCR:
-
Natural cytotoxicity receptor
- NKT:
-
Natural killer T
- TGF:
-
Transforming growth factor
- Treg:
-
Regulatory T cell
- TCR:
-
T cell receptor
- Th:
-
T helper
- TNF:
-
Tumor necrosis factor
- UC:
-
Ulcerative colitis
References
Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321.
Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimizing response to anti-TNF therapy in Crohn’s disease—algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51.
Vermeire S, Loftus EV Jr, Colombel JF, et al. Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis. 2017;11:412–424.
Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis. 2017;11:400–411.
Weigmann B, Neurath MF. Th9 cells in inflammatory bowel diseases. Semin Immunopathol. 2017;39:89–95.
Goldberg R, Prescott N, Lord GM, MacDonald TT, Powell N. The unusual suspects—innate lymphoid cells as novel therapeutic targets in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:271–283.
Page MJ, Poritz LS, Tilberg AF, Zhang WJ, Chorney MJ, Koltun WA. Cd1d-restricted cellular lysis by peripheral blood lymphocytes: relevance to the inflammatory bowel diseases. J Surg Res. 2000;92:214–221.
Treiner E. Mucosal-associated invariant T cells in inflammatory bowel diseases: bystanders, defenders, or offenders? Front Immunol. 2015;6:27.
MacDonald TT. The role of activated T lymphocytes in gastrointestinal disease. Clin Exp Allergy. 1990;20:247–252.
Monteleone G, Biancone L, Marasco R, et al. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178.
Monteleone G, Trapasso F, Parrello T, et al. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–147.
Zorzi F, Monteleone I, Sarra M, et al. Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One. 2013;8:e54562.
Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270.
Camoglio L, Te Velde AA, Tigges AJ, Das PK, Van Deventer SJ. Altered expression of interferon-gamma and interleukin-4 in inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:285–290.
Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564.
Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497.
Rovedatti L, Kudo T, Biancheri P, et al. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58:1629–1636.
Biancheri P, Di Sabatino A, Ammoscato F, et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol. 2014;44:370–385.
Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–3470.
Lamb CA, Mansfield JC, Tew GW, et al. αEβ7 integrin identifies subsets of Pro-inflammatory colonic CD4+ T lymphocytes in ulcerative colitis. J Crohns Colitis. 2017;11:610–620.
Gwela A, Siddhanathi P, Oxford IBD Cohort Investigators, et al. Th1 and innate lymphoid cells accumulate in primary sclerosing cholangitis-associated inflammatory bowel disease. J Crohns Colitis. 2017;11:1124–1134.
Di Sabatino A, Rovedatti L, Kaur R, et al. Targeting gut T cell Ca2+ release-activated Ca2+ channels inhibits T cell cytokine production and T-box transcription factor T-bet in inflammatory bowel disease. J Immunol. 2009;183:3454–3462.
Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939.
Wilson MS, Ramalingam TR, Rivollier A, et al. Colitis and intestinal inflammation in IL10−/− mice results from IL-13Rα2-mediated attenuation of IL-13 activity. Gastroenterology. 2011;140:254–264.
Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013.
Vainer B, Nielsen OH, Hendel J, Horn T, Kirman I. Colonic expression and synthesis of interleukin 13 and interleukin 15 in inflammatory bowel disease. Cytokine. 2000;12:1531–1536.
Kadivar K, Ruchelli ED, Markowitz JE, et al. Intestinal interleukin-13 in pediatric inflammatory bowel disease patients. Inflamm Bowel Dis. 2004;10:593–598.
Bernardo D, Vallejo-Díez S, Mann ER, et al. IL-6 promotes immune responses in human ulcerative colitis and induces a skin-homing phenotype in the dendritic cells and T cells they stimulate. Eur J Immunol. 2012;42:1337–1353.
Danese S, Rudziński J, Brandt W, et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut. 2015;64:243–249.
Reinisch W, Panés J, Khurana S, et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut. 2015;64:894–900.
Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346.
Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355.
Gerlach K, McKenzie AN, Neurath MF, Weigmann B. IL-9 regulates intestinal barrier function in experimental T cell-mediated colitis. Tissue Barriers. 2015;3:e983777.
Gerlach K, Hwang Y, Nikolaev A, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676–686.
Nalleweg N, Chiriac MT, Podstawa E, et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut. 2015;64:743–755.
Blom L, Poulsen BC, Jensen BM, Hansen A, Poulsen LK. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS One. 2011;6:e21695.
Seidelin JB, Bjerrum JT, Coskun M, Widjaya B, Vainer B, Nielsen OH. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128:80–85.
Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107:8017–8022.
Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657.
Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70.
Sugihara T, Kobori A, Imaeda H, et al. The increased mucosal mRNA expressions of complement C3 and interleukin-17 in inflammatory bowel disease. Clin Exp Immunol. 2010;160:386–393.
Monteleone G, Monteleone I, Fina D, et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology. 2005;128:687–694.
Fina D, Sarra M, Fantini MC, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048.
Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132.
Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861.
Biancheri P, Pender SL, Ammoscato F, et al. The role of interleukin 17 in Crohn’s disease-associated intestinal fibrosis. Fibrogenes Tissue Repair. 2013;6:13.
Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 2008;57:1682–1689.
O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805.
Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338.
Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4 + CD25-T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153.
Dominitzki S, Fantini MC, Neufert C, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4 + CD25 T cells. J Immunol. 2007;179:2041–2045.
Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4 + CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878.
Chamouard P, Monneaux F, Richert Z, et al. Diminution of circulating CD4 + CD25 high T cells in naïve Crohn’s disease. Dig Dis Sci. 2009;54:2084–2093.
Li Z, Vermeire S, Bullens D, et al. Restoration of Foxp3+ regulatory T-cell subsets and Foxp3− type 1 regulatory-like T cells in inflammatory bowel diseases during anti-tumor necrosis factor therapy. Inflamm Bowel Dis. 2015;21:2418–2428.
Saruta M, Yu QT, Fleshner PR, et al. Characterization of FOXP3 + CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281–290.
Fahlén L, Read S, Gorelik L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746.
Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–609.
Biancheri P, Giuffrida P, Docena GH, MacDonald TT, Corazza GR, Di Sabatino A. The role of transforming growth factor (TGF)-β in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev. 2014;25:45–55.
Fantini MC, Rizzo A, Fina D, et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136:1308–1316.
Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218.
Ruijing X, Mengjun W, Xiaoling Z, et al. Jα33+ MAIT cells play a protective role in TNBS induced intestinal inflammation. Hepatogastroenterology. 2012;59:762–767.
Serriari NE, Eoche M, Lamotte L, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266–274.
Haga K, Chiba A, Shibuya T, et al. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol. 2016;31:965–972.
Tominaga K, Yamagiwa S, Setsu T, et al. Possible involvement of mucosal-associated invariant T cells in the progression of inflammatory bowel diseases. Biomed Res. 2017;38:111–121.
Hiejima E, Kawai T, Nakase H, et al. Reduced Numbers and proapoptotic features of mucosal-associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1529–1540.
Saubermann LJ, Beck P, De Jong YP, et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119–128.
Numata Y, Tazuma S, Ueno Y, Nishioka T, Hyogo H, Chayama K. Therapeutic effect of repeated natural killer T cell stimulation in mouse cholangitis complicated by colitis. Dig Dis Sci. 2005;50:1844–1851.
Ueno Y, Tanaka S, Sumii M, et al. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of v alpha 14 natural killer T cells in mice. Inflamm Bowel Dis. 2005;11:35–41.
Hornung M, Farkas SA, Sattler C, Schlitt HJ, Geissler EK. DX5+ NKT cells induce the death of colitis-associated cells: involvement of programmed death ligand-1. Eur J Immunol. 2006;36:1210–1221.
Shibolet O, Alper R, Zolotarov L, et al. The role of intrahepatic CD8+ T cell trapping and NK1.1+ cells in liver-mediated immune regulation. Clin Immunol. 2004;111:82–92.
Shibolet O, Kalish Y, Klein A, et al. Adoptive transfer of ex vivo immune programmed NKT lymphocytes alleviates immune-mediated colitis. J Leukoc Biol. 2004;75:76–86.
Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638.
Perera L, Shao L, Patel A, et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm Bowel Dis. 2007;13:298–307.
Grose RH, Thompson FM, Baxter AG, Pellicci DG, Cummins AG. Deficiency of invariant NK T cells in Crohn’s disease and ulcerative colitis. Dig Dis Sci. 2007;52:1415–1422.
Fuss IJ, Joshi B, Yang Z, et al. IL-13Rα2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut. 2014;63:1728–1736.
Fuchs A, Vermi W, Lee JS, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769–781.
Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375.
Powell N, Walker AW, Stolarczyk E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor + innate lymphoid cells. Immunity. 2012;37:674–684.
Geremia A, Arancibia-Cárcamo CV, Fleming MP, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133.
Powell N, Lo JW, Biancheri P, et al. Interleukin 6 increases production of cytokines by colonic innate lymphoid cells in mice and patients with chronic intestinal inflammation. Gastroenterology. 2015;149:456–467.
Bernink JH, Peters CP, Munneke M, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229.
Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970.
Bernink JH, Krabbendam L, Germar K, et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43:146–160.
Lim AI, Menegatti S, Bustamante J, et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213:569–583.
Oka A, Ishihara S, Mishima Y, et al. Role of regulatory B cells in chronic intestinal inflammation: association with pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20:315–328.
Ansary MM, Ishihara S, Oka A, et al. Apoptotic cells ameliorate chronic intestinal inflammation by enhancing regulatory B-cell function. Inflamm Bowel Dis. 2014;20:2308–2320.
Li Z, Vermeire S, Bullens D, et al. Anti-tumor necrosis factor therapy restores peripheral blood B-cell subsets and CD40 Expression in inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21:2787–2796.
Di Sabatino A, Rosado MM, Cazzola P, et al. Splenic function and IgM-memory B cells in Crohn’s disease patients treated with infliximab. Inflamm Bowel Dis. 2008;14:591–596.
Keren DF, Appelman HD, Dobbins WO 3rd, et al. Correlation of histopathologic evidence of disease activity with the presence of immunoglobulin-containing cells in the colons of patients with inflammatory bowel disease. Hum Pathol. 1984;15:757–763.
Giuffrida P, Pinzani M, Corazza GR, Di Sabatino A. Biomarkers of intestinal fibrosis—one step towards clinical trials for stricturing inflammatory bowel disease. United European Gastroenterol J. 2016;4:523–530.
Uo M, Hisamatsu T, Miyoshi J, et al. Mucosal CXCR4+ IgG plasma cells contribute to the pathogenesis of human ulcerative colitis through FcγR-mediated CD14 macrophage activation. Gut. 2013;62:1734–1744.
Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Epithelial deposition of immunoglobulin G1 and activated complement (C3b and terminal complement complex) in ulcerative colitis. Gastroenterology. 1990;98:1264–1271.
Gordon JN, Pickard KM, Di Sabatino A, et al. Matrix metalloproteinase-3 production by gut IgG plasma cells in chronic inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:195–203.
Giuffrida P, Biancheri P, MacDonald TT. Proteases and small intestinal barrier function in health and disease. Curr Opin Gastroenterol. 2014;30:147–153.
Cupi ML, Sarra M, Marafini I, et al. Plasma cells in the mucosa of patients with inflammatory bowel disease produce granzyme B and possess cytotoxic activities. J Immunol. 2014;192:6083–6091.
Sato T, Nakai T, Tamura N, et al. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 2005;54:1254–1262.
Wang Z, Wang Z, Wang J, Diao Y, Qian X, Zhu N. T-bet-expressing B cells are positively associated with Crohn’s disease activity and support Th1 inflammation. DNA Cell Biol. 2016;35:628–635.
Sandborn WJ, Cyrille M, Hansen MB, et al. Efficacy and safety of abrilumab in subjects with moderate to severe ulcerative colitis: results of a phase 2b, randomised, double-blind, multiple-dose, placebo-controlled study. Gastroenterology. 2017;152:S198.
Sandborn WJ, Cyrille M, Hansen MB, et al. Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. Gastroenterology. 2017;152:S598.
Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–318.
Vermeire S, Sandborn WJ, Danese S, et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:135–144.
Sandborn WJ, Lee SD, Tarabar D et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut. 10/5/2017 [Epub ahead of print].
Schreiber S, Nikolaus S, Malchow H, et al. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active Crohn’s disease. Gastroenterology. 2001;120:1339–1346.
Miner PB Jr, Wedel MK, Xia S, Baker BF. Safety and efficacy of two dose formulations of alicaforsen enema compared with mesalazine enema for treatment of mild to moderate left-sided ulcerative colitis: a randomized, double-blind, active-controlled trial. Aliment Pharmacol Ther. 2006;23:1403–1413.
Leiper K, Martin K, Ellis A, et al. Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut. 2011;60:1520–1526.
Sandborn WJ, Colombel JF, Frankel M, et al. Anti-CD3 antibody visilizumab is not effective in patients with intravenous corticosteroid-refractory ulcerative colitis. Gut. 2010;59:1485–1492.
van der Woude CJ, Stokkers P, van Bodegraven AA, et al. Phase I, double-blind, randomized, placebo-controlled, dose-escalation study of NI-0401 (a fully human anti-CD3 monoclonal antibody) in patients with moderate to severe active Crohn’s disease. Inflamm Bowel Dis. 2010;16:1708–1716.
Vossenkämper A, Hundsrucker C, Page K, et al. A CD3-specific antibody reduces cytokine production and alters phosphoprotein profiles in intestinal tissues from patients with inflammatory bowel disease. Gastroenterolog.. 2014;147:172–183.
Creed TJ, Probert CS, Norman MN, et al. Basiliximab for the treatment of steroid-resistant ulcerative colitis: further experience in moderate and severe disease. Aliment Pharmacol Ther. 2006;23:1435–1442.
Sands BE, Sandborn WJ, Creed TJ, et al. Basiliximab does not increase efficacy of corticosteroids in patients with steroid-refractory ulcerative colitis. Gastroenterology. 2012;143:356–364.
Van Assche G, Sandborn WJ, Feagan BG, et al. Daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor (CD25), for the treatment of moderately to severely active ulcerative colitis: a randomised, double blind, placebo controlled, dose ranging trial. Gut. 2006;55:1568–1574.
Allez M, Skolnick BE, Wisniewska-Jarosinska M, Petryka R, Overgaard RV. Anti-NKG2D monoclonal antibody (NNC0142-0002) in active Crohn’s disease: a randomised controlled trial. Gut. 2017;66:1918–1925.
Reinisch W, de Villiers W, Bene L, et al. Fontolizumab in moderate to severe Crohn’s disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm Bowel Dis. 2010;16:233–242.
Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700.
Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960.
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02407236?term=ustekinumab&cond=Ulcerative+Colitis&rank=1; 2017.
Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Interleukin-12 deficiency is permissive for angiogenesis in UV radiation-induced skin tumors. Cancer Res. 2007;67:3785–3793.
Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology. 2017;153:77–86.
Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–1709.
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02891226?term=mirikizumab&cond=Crohn+Disease&rank=1; 2017.
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02589665?term=mirikizumab&cond=ulcerative+colitis&rank=1; 2017.
Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–996.
Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736.
Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomized placebo-controlled trials. Gut. 2017;66:1049–1059.
Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275.
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02782663?term=ABT494&cond=Crohn+Disease&rank=2; 2017.
Popp V, Gerlach K, Mott S, et al. Rectal delivery of a DNAzyme that specifically blocks the transcription factor GATA3 and reduces colitis in mice. Gastroenterology. 2017;152:176–192.
Colombel JF, Rutgeerts P, Malchow H, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut. 2001;49:42–46.
Schreiber S, Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461–1472.
Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473–1482.
Monteleone G, Neurath MF, Ardizzone S, et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015;372:1104–1113.
Monteleone G, Di Sabatino A, Ardizzone S, et al. Impact of patient characteristics on the clinical efficacy of mongersen (GED-0301), an oral Smad7 antisense oligonucleotide, in active Crohn’s disease. Aliment Pharmacol Ther. 2016;43:717–724.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Giuffrida, P., Corazza, G.R. & Di Sabatino, A. Old and New Lymphocyte Players in Inflammatory Bowel Disease. Dig Dis Sci 63, 277–288 (2018). https://doi.org/10.1007/s10620-017-4892-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4892-4