Abstract
Cryopreserved (CryoPA) and Glycerol-preserved (GPA) skin allografts are commonly used in the treatment of severe burn injuries. However, comparable data on their differences in clinical outcome is scarce. This retrospective review aims to study the effect of allograft viability on clinical outcomes. The records of 48 severe burn patients who either received CryoPA or GPA were reviewed. Key burn mortality determinants were used to match the 2 groups. Clinical outcomes such as mortality rate (MR) and the length of hospital stay (LOS) were obtained. A separate in vitro comparison included histological assessments and the use of tetrazolium reductase activity to compare tissue viability. Both groups showed a comparable profile in burn mortality determinants. Patients who received CryoPA had a lower MR of 25% compared to 34.8% (P = 0.250) in the GPA group and a lower LOS of 39.2–45.9 days (P = 0.730), respectively. The histological structural integrity was found to be well preserved with both methods although CryoPA was confirmed to be the more viable product (P < 0.05). The lower MR associated with CryoPA cannot be totally ignored. However, the mechanism through which viable skin allografts improves MR of severe burns patients remains to be elucidated.
Similar content being viewed by others
Introduction
Skin allografts derived from human cadaver donors are widely used in the treatment of massive burn injuries due to the paucity of available autologous donor skin in such patients. They offer many distinct advantages over conventional dressings. The allografts provide mechanical and physiologic barriers that reduce protein and water loss from the wound surface, protect wounds from bacterial contamination, prepare recipient granulation beds for autografting and increase the comfort and well-being of the patient (May et al. 1984; Burd and Chiu 2005; Druecke et al. 2002; Wachtel et al. 1979).
Allograft cadaver skin was first applied on burn wounds in 1881 (Girdner 1881; Rogers 1951) and their importance as a temporary biological dressing in the treatment of burns has become well established over the last 2 decades (Vloemans et al. 2002a, b; Moerman et al. 2002; Blome-Eberwein et al. 2002). Fresh cadaver allografts are considered the gold standard skin substitute for temporary closure of large full-thickness burn wounds but their use is severely impeded by their inadequate availability (Kearney 1998; Greenleaf and Hansbrough 1994). The American Association of Tissue Banks (AATB) states that the viability of skin is an essential prerequisite of good quality grafts for the functional closure of wounds (Baxter 1985). Maintenance of cell viability and structural integrity have been postulated to be vital for the engraftment and neovascularization of allograft skin (Bravo et al. 2000). However, the availability of fresh allograft do not coincide with occurrence of severe burns and the shelf life of fresh allograft at normal refrigeration temperature is limited (DeBono et al. 1998), thus skin graft preservation for the purpose of delayed application has become a vital tool in burn therapeutics. Preserved allograft allows for a longer storage period and allows more time for the sample to be assessed microbiologically to certify its sterility and viability (Bravo et al. 2000; Castagnoli et al. 2003; Başaran et al. 2006).
There are 2 main methods of allograft preservation. Cryopreserved allograft (CryoPA) is frozen in liquid nitrogen at −180°C and Glycerol preserved allograft (GPA) is stored in 85% glycerol at 4°C. Cryopreservation has been shown to maintain cell viability up to 50% (Bravo et al. 2000) but glycerol preservation results in a non-viable skin which is less antigenic (Richters et al. 1997) with anti-bacterial and anti-viral properties (Van Baare et al. 1994; Hettich et al. 1994). Many skin banks employ cryopreservation but the cost and complexity of cryopreservation processes have encouraged some skin banks to adopt glycerolization of cadaveric skin (Mackie 1997; De Backere 1994; Vuola and Pipping 2002). Considerable controversy still exists over the viability of preserved skin allografts and whether viability is a prerequisite for its function as a biologic dressing (Aggerwal et al. 1985). Although there have been some studies comparing allograft viability and transplantation performance (Ben-Bassat et al. 2000, 2001), there has been no previous study comparing the actual patient outcomes between the use of CryoPA and GPA. This retrospective review aims to compare the clinical outcome between severe burn patients treated with CryoPA and GPA as well as compare the tissue viability and histomorphology of these two types of allograft in vitro.
Methods
Preservation protocols
Skin samples harvested from cadaveric donors were rinsed with 0.025% sodium hypochlorite mixed in phosphate buffer saline (PBS) to remove excess lubricant as well as dead skin cells. The specimens were then placed under 4°C storage in DMEM for 10 days as fresh skin tissues. After 10 days, skin samples to be cryopreserved were soaked in DMEM with 10% DMSO to allow penetration of the cryoprotectant into the tissues. This is followed by freezing at a slow rate of −1 to −5°C/min using a programmable control rate freezer. At −100°C, the allograft is transferred to a −150°C CFC-free ultralow freezer. Before application, rapid tissue thawing was carried out in a pre-warm (37°C) 0.9% saline solution, and the tissue was rinsed thoroughly three times in the saline solution.
Glycerolised skin allografts (GPA) were obtained from the Euro Skin Bank and stored at 4°C. GPA specimens that have been preserved for 1–2 years were used in the experiments. Before application, the specimens were rehydrated by soaking in saline solution at room temperature for 10 min followed by extensive rinsing in saline solution.
Viability evaluation of human skin preserved in glycerol or in liquid nitrogen
Quantitative analysis of tissue viability between the different skin allografts was performed by measurement of the tetrazolium reductase activity as described by Hershey et al. (1958) and Castagnoli et al. (2003). This assay was chosen for our study because of its reproducibility and it allows for cellular viability testing of intact tissues without disrupting the structural integrity of the tissue. Tetrazolium salts are reduced by mitochondrial enzymes in viable cells into formazan pigments. The quantity of pigment produced by metabolically active cells can then be determined spectrophotometrically. The obtained value minus the negative control is proportional to the cumulative metabolic activity of the cells in the tissue and thus may be construed as equating to its viability. Our assay varied from Hershey’s in that 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) solution was chosen for this study instead of 3-(4,5-dimethylthiazol-2-yl)-2,5-(diphenyltetrazolium bromide (MTT) because the insoluble formazan product of MTT reduction required additional steps to dissolve the crystals before optical absorbance reading at 570 nm while the formazan product of MTS is soluble in tissue culture medium.
Skin biopsies (n = 20) were obtained from CryoPA and GPA allografts and compared against negative controls which were devitalized by boiling. Skin disks of 6 mm diameters were obtained by punch-biopsy (Stiefel Laboratories, Ireland) and placed dermal side down in a 24-well tissue culture plate. To ensure uniformity between the samples, each sample was weighed and homogeneity of all samples were assessed to ensure comparable permeation of the salts into the tissue. Post-cryopreserved samples was assayed immediately after undergoing post-thawing cryoprotectant dilution and rinsing. MTS solutions (0.5 mg/ml) were added to the transport medium and the samples were then incubated at 37°C, in an atmosphere of 5% CO2/air. After an overnight incubation, the solution was then read on a spectrophotometer (492 nm). The viability index of a skin sample was expressed as the ratio of its Optical Density (OD) to its weight.
The mean viability index was calculated. The percentage viability index (PVI) of the GPA is the mean viability normalised to that of the cryopreserved allograft.
Comparison of histological specimens
Some cryopreserved and glycerolized samples (n = 8) were processed for histological analysis. Tissue samples were washed with phosphate buffered saline (PBS), fixed in 10% buffered formalin and paraffin-embedded before the 5 mm sections were stained with hematoxylin-eosin. Histomorphological analysis was performed to verify the integrity of tissue architecture of both allografts. The specimens were examined microscopically and scored by a set of five histological criteria, each marked 0–2 (Table 1). The total score for each specimen was calculated by adding the individual scores of all the criteria.
Retrospective case–control study
Between April 2001 and October 2007, a total of 1,483 burn patients were admitted to the Singapore General Hospital (SGH) burns centre. A total of 56 patients met the inclusion criteria of this study: 20 years old or older, deep dermal to full thickness bums greater than 30% total body surface area (TBSA) and treated with either CryoPA or GPA. 6 patients were omitted from the study after further stratification from cross-tabulation with age and TBSA to ensure that both study groups were comparable. Patients with co-morbidities were excluded (Fig. 1). Most of the patients (n = 38) had undergone early eschar excision with skin autograft/allograft coverage within 72 h and complete excision of burn wounds within 7 days. The remaining 10 patients did not undergo early burns excision as they were transferred from overseas. All the patients were treated according to a similar ward protocol in the burns centre and patients with conditions or diseases (diabetes, etc.) known to affect burn treatment were excluded from the study.
The selected population was divided into two groups, consisting of patients who were treated with CryoPA and patients treated with GPA solely. Patients who received both CryoPA and GAP were excluded from the study. Key determinants of burns mortality were used to match the 2 groups in comparison, namely: age, TBSA, presence of inhalational injury and intubation, APACHE II scores on admission and early eschar excision. Other factors such as resuscitation techniques, type of ventilator, nutrition and ward care were comparable for both groups as these modalities did not differ significantly during the study period. Clinical outcomes measured were mortality rate (MR) and the length of hospital stay (LOS).
The Acute physiologic and chronic health evaluation (APACHE) II is widely used to predict patient outcome in the intensive care unit (Knaus et al. 1985). Wong et al. (2002) reported that the APACHE II score on admission was an important predictor of burn mortality. It involves assigning numerical values (0–4 with high scores indicating more severe illness) to 12 clinical and biochemical parameters: temperature, mean arterial blood pressure, heart rate, respiratory rate, oxygenation, arterial ph, serum sodium, potassium and creatinine, WBC and GCS. The combined score from these 12 parameters makes up the Acute Physiology Score (APS) of APACHE II. Points are also assigned for age group and preexisting illness. Combined scores below 10 suggest relatively mild illness while score above 15 indicate moderate to severe illness. According to the APACHE II definition, scores were calculated based on the worst physiologic parameters within the first 24 h following hospital admission. APACHE II scores between the 2 study groups were evaluated to determine if they were evenly matched.
The retrospective statistical analysis was performed using Chi-square and Mann–Whitney test for patients’ profile and clinical outcome.
Results
Viability evaluation of cryopreserved and glycerolized skin
Figure 2 shows that there were no significant differences between the sample disk weights of the various study groups (including the negative controls). Therefore it can be concluded that the MTS tissue viability analysis was carried out on approximately uniform and homogenous samples.
The mean viability score obtained from immediate post-thaw cryopreserved samples was normalised to an index of 100% for comparison with the GPA sample. GPA is observed to have a viability reading of 10.27 ± 7.25% relative to post-thaw CryoPA (Fig. 3). This was statistically significant.
Histological analysis of cryopreserved and glycerolized skin
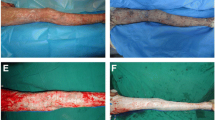
Histologically, the cryopreserved and glycerolized skin specimens were analysed. Although the samples showed some signs of cellular damage after both preservation methods, histological analysis performed on both grafts showed good preservation of the tissue architecture. The mean microanalysis scores of CryoPA was comparable to GPA (Fig. 4) although the glycerolized stratum corneum was observed to have a higher fragmentation rate. The macroanalysis scores of CryoPA is higher that GPA (Fig. 5) as GPA was opined to be less pliable and it did not resemble fresh skin as well as thawed CryoPA.
In comparison, histology analysis was also conducted on the negative controls for comparison. The skin architecture was completely destroyed. The epidermal layer was completely detached from the underlying dermis and the cells were severely damaged (Fig. 6c, d). The reticular dermis area became smooth and compact with no rete ridges. The sections were stained deep purple due to the denatured proteins and structures.
Retrospective-statistical comparison of clinical outcomes
Among all the patients admitted to the burns unit during the period 2001–2007, 48 patients met the inclusion criteria. Table 2 demonstrates the profile of the 2 groups in comparison. From Table 2, it can be observed that the two study groups had similar profiles and were comparable for key burns mortality determinants such as age, TBSA, full thickness burns, APACHE II scores, smoke inhalation, intubation and early excision. Table 3 detailed the statistical analysis of the clinical performances of cryopreserved and glycerolized skin.
Although the comparison of the clinical outcomes between CryoPA and GPA was not statistically significant, mortality in the CryoPA group was seen to be lower compared to the GPA group (25% vs. 34.8%, P = 0.25). The mean LOS for CryoPA and GPA was 39.20 and 45.93 days (P = 0.730), respectively.
Discussion
Over the last few decades, fresh viable cadaveric allografts have proven to be a very effective alternate material to cover tangentially excised deep second or third degree-burns when insufficient amounts of autografts are present. Their acceptance as a temporary cover, coupled with the advent of early excision and grafting for severe burns has led to an increase in their demand and the research and establishment of various procedures for skin preservation (Kearney 1998; Herndon et al. 1989). Two methods of skin preservation have proven useful: cryopreservation and glycerol preservation. Cryopreservation aims to preserve the viable properties of fresh cadaver skin but the process is costly and complex. Glycerolization is simple and less expensive but the allograft is considered nonviable. The American Association of Tissue Banks indicates that the viability of skin is an essential prerequisite for good quality grafts and for the functional closure of wounds (Baxter 1985). It is widely believed that no skin substitute is able to approximate the biological properties of viable human skin. However, considerable controversy still exists over the viability of preserved allografts and whether viability is necessary for its function as a biologic dressing (Aggerwal et al. 1985; Bondoc and Burke 1971; Wachtel et al. 1979).
There have been some reports comparing the clinical transplantation performance between CryoPA and GPA (Aggerwal et al. 1985; Ben-Bassat et al. 2001; Wachtel et al. 1979). However, there is no study comparing the actual clinical outcomes in patients treated with either GPA or CryoPA. A historical appraisal of the use of CryoPA (1979–1981) and GPA (1998–2000) in the treatment of partial-thickness burns indicated a reduction in the need for second autografting in the group treated with GPA (Vloemans et al. 2002a, b). Vloeman et al. commented that the results were not statistically significant and could have been attributed to the factors such as improvement in health and social welfare, differences in treatment protocols and differences in graft properties. However, he also hypothesized that the diminished immunogenicity of the non-viable GPA could have resulted in the moderation of the local inflammation process and contributed to a better wound healing. Cryopreservation is able to preserve allograft viability to a certain extent but interaction between the host and donor cells may potentially trigger early rejection. Early graft rejection before epithelisation of the wound can cause further deterioration of the wound bed and possible increased risk in infection and scarring. Advantages of treatment with GPA include its antibacterial (Obeng et al. 2001; Pianigiani et al. 2006) and virucidal effects and a delay in graft rejection due to decreased immunogenicity. In vitro immunogenenicity of glycerolised skin had been compared to untreated skin by coculture of peripheral T-cells with allogeneic treated skin cells. The results indicated an inflammatory process that is mediated by infiltrating host monocytes rather than a rejection process mediated by T cells (Richters et al. 1996, 1999). Other studies on GPA have also demonstrated a consistent graft take in patients, resulting in greater than 95% epithelization at 6 weeks with less than 2% graft failure (Ben-Bassat et al. 2000). The glycerolization process has also been shown to reduce intracellular virus infectivity and thus minimising disease and infection transmission (Marshall et al. 1995; Mackie 2002). Furthermore, to gain more insight into the clinical practice of GPA for the management of burn injuries, a postal survey of 62 burn centers that have received GPA over the past 5 years demonstrated a general concensus that GPA had performed well in clinical practice with few complications (Eade 1958). Table 5 summarizes the differences and similarities between both allografts.
Our study aims to re-examine the necessity of preserving allogenic skin viability for the treatment of severe burns by examining the clinical outcomes. We first compared cell viability of CryoPA and GPA using in vitro assessments where we demonstrated that GPA is indeed a much less viable product compared to CryoPA. However, the histological architecture of both CryoPA and GPA are relatively well preserved. From our retrospective analysis, it appears that the risk of mortality in patients treated with CryoPA group is lower when compared to the GPA group (25% vs. 34.8%, P = 0.250) (Table 3). The lower mortality risk with the use of CryoPA is also demonstrated when only patients with early burns excision were compared in the 2 groups. (19.0% versus 35.3%, P = 0.258) (Table 4). Similarly the overall LOS is also lower in the group treated with CryoPA (39.2 days vs. 45.93 days, P = 0.730). Although the results were not statistically significant due to the eventual small sample size, these results suggest that viable allogenic skin cells may play an important role in improving severe burn patients’ outcome, especially mortality risk, given the relatively their low P values. We hypothesize that the lower mortality and LOS when treated with the more viable graft could be contributed by 3 factors. Firstly, the more viable CryoPA may function as a superior biological dressing due to its better transplantation performance. Secondly, the more viable CryoPA is better able to stimulate neovascularisation and thus enhance wound healing. Thirdly, the viable allograft cells in CryoPA could have a role in the immunomodulation of the systemic response in severe burns (Table 5).
Cadaveric skin allografts have generally been considered to be effective in protecting wounds from bacterial contamination and the reduction of water, electrolyte and protein losses. Non-viable GPA is also able to perform this function as it is able to retain the morphological structure of skin and perform its barrier function. The non-viable allograft adheres to the wound as a ‘collagen prosthesis’ but does not undergo true chemical bonding with fibrin of the wound. Eade observed that skin coverage with fresh viable or preserved non-viable skin grafts eliminated the dead space existing on the surface of burn wounds and permitted granulation tissue to destroy surface bacteria rapidly (Cinamon et al. 1993). Successful take of the graft would effectively eliminate the dead space existing on the surface, thus allowing the graft to perform its functions as a biologic dressing better. Cinamon et al. demonstrated that CryoPA had a better transplantation performance compared to GPA in an immunocompetent mouse recipient model (O’Donoghue and Zarem 1971). Primary take was evaluated via macroscopic evaluation (adherence to wound bed, skin colour and pliability) and histological evaluation. The cryopreserved skin grafts performed significantly better than glycerolised skin even after a transplantation period as short as 4 days. The difference became even more significant after 7 days of grafting. Both forms of preserved allografts provided a less successful product than fresh cadaver skin, therefore demonstrating that viability of the allografts may effect better transplantation performance.
Cadaveric skin allografts have also been known to improve wound healing by promoting neovascularisation of the recipient bed through engraftment. O’Donogue et al. observed that fresh skin grafts were clearly superior in stimulating neovascularisation in the wound bed than preserved skin grafts (freeze-thawed and lysophilized) (O’Donoghue and Zarem 1971). The maintenance of cell viability and structural integrity was postulated to be vital for this effect. Faster wound healing decreases release of inflammatory mediators and bacterial colonization of wounds. This, in turn, attenuates the systemic inflammatory response syndrome (SIRS) hence reducing the occurrence of metabolic derangements, sepsis and multi-organ failure (MOF).
The lower mortality risk associated with the use of CryoPA in our current study can be partly attributed to the enhanced wound healing effects described above and possibly due to other systemic factors. We hypothesize that viable allograft cells could have attenuated the systemic inflammatory response. Depressed Th1 and exaggerated Th2 cytokine responses were demonstrated in burn patients and these two observations were related to the increased susceptibility to SIRS, sepsis and MOF in such patients. Wolf et al. previously demonstrated that IGF-1/IGFBP-3 treatment in burn patients could reversed the postburn Th2 shift and might partially restore immune function (Miller et al. 2007; Wolf et al. 2004). Jerschke et al. showed that insulin treatment can attenuate the inflammatory response by decreasing the pro-inflammatory and increasing the anti-inflammatory cascade, thus decreasing morbidity and mortality in critical conditions (Jeschke et al. 2004). Other cytokines that are reported to have a role in immunomodulation are interleukin-12 (IL-12) and interferon-γ (IFN-γ) (Wolf et al. 2004). Our hypothesis is that viable allograft cells can stimulate the production of favourable cytokines that immunomodulates the Th1/Th2 balance and reduces the risk of progression to SIRS and MOF. The exact mechanism of this is still unclear but these initial results certainly merit further study by means of a prospective, comparative trial.
Conclusion
Glycerolised allografts have been shown to be less viable than cryopreserved allografts although there is good preservation of tissue architecture with both methods. The clinical outcomes between patients treated with CryoPA and GPA were compared and it was observed that viable allograft cells may play a role in decreasing mortality risk and LOS in severe burn patients. While it could not be confirmed that CryoPA is superior to GPA statistically for the key clinical outcomes, the better clinical outcomes associated with the former cannot be totally ignored. Therefore, the mechanism through which viability of temporary skin allograft improves clinical outcomes of severe burns patients remains to be elucidated.
References
Aggerwal GJ, Baxter CR, Diller KR (1985) Cryopreservation of skin. An assessment of current clinical application. J Burn Care Rehabil 6:469–476
Başaran O, Ozdemir H, Kut A, Sahin FI, Deniz M, Sakallioğlu EA, Haberal MA (2006) Effects of different preservation solutions on skin graft epidermal cell viability and graft performance in a rat model. Burns 32(4):423–429
Baxter CR (1985) Skin banking in the USA. J Burn Care Rehabil 6:322
Ben-Bassat H, Chaouat M, Zumai E, Segal N, Cinamon U, Ron M, Wexler MR, Eldad A (2000) The Israel national skin bank: quality assurance and graft performance of stored tissues. Cell Tissue Bank 1(4):303–312
Ben-Bassat H, Chaouat M, Segal N, Zumai E, Wexler MR, Eldad A (2001) How long can cryopreserved skin be stored to maintain adequate graft performance? Burns 27:425–431
Blome-Eberwein S, Jester A, Kuentscher M, Raff T, Germann G, Pelzer M (2002) Clinical practice of glycerol preserved allograft skin coverage. Burns 28(Suppl 1):S10–S12
Bondoc CC, Burke JF (1971) Clinical experience with viable frozen human skin and a frozen skin bank. Ann Surg 174(3):371–382
Bravo D, Rigley TH, Gibran N, Strong DM, Newman-Gage H (2000) Effect of storage and preservation methods on viability in transplantable human skin allografts. Burns 26(4):367–378
Burd A, Chiu T (2005) Allogenic skin in the treatment of burns. Clin Derm 23:376–387
Castagnoli C, Alotto D, Cambieri I, Casimiri R, Aluffi M, Stella M, Alasia ST, Magliacani G (2003) Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolioum salt assay. Burns 29(8):759–767
Cinamon U, Eldad A, Chaouat M, Wexler RM, Israeli A, Zagher U, Ben-Bassat H (1993) A simplified testing system to evaluate performance after transplantation of human skin preserved in glycerol or in liquid nitrogen. J Burn Care Rehabil 14:435–439
de Backere AC (1994) Euro skin bank: large scale skin-banking in Europe based on glycerol-preservation of donor skin. Burns 20(Suppl 1):S4–S9
DeBono R, Rao GS, Berry RB (1998) The survival of human skin stored by refrigeration at 4 degrees C in McCoy’s 5A medium: does oxygenation of the medium improve storage time? Plast Reconstr Surg 102(1):78–83
Druecke D, Steinstraesser L, Homann HH, Steinau HU, Vogt PM (2002) Current indications for glycerol-preserved allografts in the treatment of burn injuries. Burns 28(Suppl 1):S26–S30
Eade GC (1958) Relationship between granulation tissue, bacteria, and skin grafts in burned patients. Plast Reconstr Surg 22:42–55
Girdner JH (1881) Skin grafting with graft taken from the dead subject. Med Rec (NY) 20:119–120
Greenleaf G, Hansbrough JF (1994) Current trends in the use of allograft skin for patients with burns and reflections on the future of skin banking in the United States. J Burn Care Rehabil 15(5):428–431
Herndon DN, Barrow RE, Rutan RL, Rutan TC, Desai MH, Abston S (1989) A comparison of conservative versus early excision. Ann Surg 209:547–553
Hershey FB, Cruickshank CN, Mullins LI (1958) The quantitative reduction of 2, 3, 5-triphenyl tetrazolium chloride by skin in vitro. J Histochem Cytochem 6(3):191–196
Hettich R, Ghofrani A, Hafemann B (1994) The immunogenicity of glycerol-preserved donor skin. Burns 20(I):S71–S76
Jeschke MG, Klein D, Herndon DN (2004) Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg 239(4):553–560
Kearney JN (1998) Quality issues in skin banking: a review. Burns 24(4):299–305
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Mackie DP (1997) The Euro skin bank: development and application of glycerol-preserved allografts. J Burn Care Rehabil 18:S7–S9
Mackie D (2002) Postal survey on the use of glycerol-preserved allografts in clinical practice. Burns 28(Suppl 1):S40–S44
Marshall L, Ghosh MM, Boyce SG, MacNeil S, Freedlander E, Kudesia G (1995) Effect of glycerol on intracellular virus survival: implications for the clinical use of glycerol-preserved cadaver skin. Burns 21(5):356–361
May SR, Still JM, Atkinson WB (1984) Recent developments in skin banking, and the clinical use of cryopreserved skin. J Med AssocGa 73:233
Miller AC, Rashid RM, Elamin EM (2007) The “T” in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma 63(6):1407–1417
Moerman E, Middelkoop E, Mackie D, Groenevelt F (2002) The temporary use of allograft for complicated wounds in plastic surgery. Burns 28(Suppl 1):S13–S15
O’Donoghue MN, Zarem HA (1971) Stimulation of neovascularization–comparative efficacy of fresh and preserved skin grafts. Plast Reconstr Surg 48(5):474–478
Obeng MK, McCauley RL, Barnett JR, Heggers JP, Sheridan K, Schutzler SS (2001) Cadaveric allograft discards as a result of positive skin cultures. Burns 27:267–271
Pianigiani E, Risulo M, Ierardi F, Sbano P, Andreassi L, Fimiani M et al (2006) Prevalence of skin allograft discards as a result of serological and molecular microbiological screening in a regional skin bank in Italy. Burns 32:348–351
Richters CD, Van Pelt AM, Van Gelderop E (1996) Migration of rat dendritic cells. J Leukoc Biol 60:317–322
Richters CD, Hoekstra MJ, van Baare J, du Pont JS, Kamperdijk EW (1997) Immunogenicity of glycerol-preserved human cadaver skin in vitro. J Burn Care Rehabil 18(3):228–233
Richters CD, van Gelderop E, du Pont JS, Hoekstra MJ, Kreis RW, Kamperdijk EW (1999) Migration of dendritic cells to the draining lymph node after allogeneic or congeneic rat skin transplantation. Transplantation 67(6):828–832
Rogers BO (1951) Guide and bibliography for research into the skin homograft problem. Plast Reconstr Surg 7:169
Van Baare J, Buitenwerf J, Hoekstra MJ, du Pont JS (1994) Virucidal effect of glycerol as used in donor skin preservation. Burns 20(Suppl 1):S77–S80
Vloemans AF, Schreinemachers MC, Middelkoop E, Kreis RW (2002) The use of glycerol-preserved allografts in the Beverwijk Burn Centre: a retrospective study. Burns 28(Suppl 1):S2–S9
Vloemans AF, Middelkoop E, Kreis RW (2002) A historical appraisal of the use of cryopreserved and glycerol-preserved allograft skin in the treatment of partial thickness burns. Burns 28(Suppl 1):S16–S20
Vuola J, Pipping D (2002) Maintaining a glycerolized skin bank—a practical approach. Burns 28(Suppl 1):S31–S33
Wachtel TL, Ninnemann J, Fisher JC, Frank HA, Inancsi W (1979) Viability of frozen allografts. Am J Surg 138(6):783–787
Wolf SE, Woodside KJ, Ramirez RJ, Kobayashi M, Suzuki F, Herndon DN (2004) Insulin-like growth factor-I/insulin-like growth factor binding protein-3 alters lymphocyte responsiveness following severe burn. J Surg Res 117(2):255–261
Wong TH, Tan BH, Ling ML, Song C (2002) Multi-resistant Acinetobacter baumannii on a burns unit–clinical risk factors and prognosis. Burns 28(4):349–357
Conflict of interest
All the authors have no conflicts of interest that could inappropriately influence the above work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kua, E.H.J., Goh, C.Q., Ting, Y. et al. Comparing the use of glycerol preserved and cryopreserved allogenic skin for the treatment of severe burns: differences in clinical outcomes and in vitro tissue viability. Cell Tissue Bank 13, 269–279 (2012). https://doi.org/10.1007/s10561-011-9254-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-011-9254-4