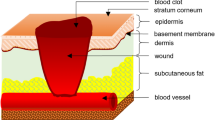
Abstract
Exogenous electrical stimulation (ES) has been investigated as a therapy for chronic wounds, as the skin produces currents and electrical fields (EFs) during wound healing. ES therapies operate by applying small EFs to the skin to mimic the transepithelial potentials that occur during the granulation phase of wound healing. Here, we investigated the effect of short duration (10 min) ES on the migration of HDFs using various magnitudes of physiologically relevant EFs. We modeled cutaneous injury by culturing HDFs in custom chambers that allowed the application of ES and then performed timelapse microscopy on a standard wound model. Using MATLAB to process cell coordinate data, we determined that the cells were migrating randomly and fit mean squared displacement data to the persistent random walk equation using nonlinear least squares regression analysis. Results indicated that application of 25–100 mV/mm DC EFs to HDFs on either uncoated or FN-coated surfaces demonstrated no significant changes in viability or proliferation. Of significance is that the HDFs increased random migration behavior under some ES conditions even after 10 min, providing a mechanism to enhance wound healing.






Similar content being viewed by others
References
Anglen, J. The clinical use of bone stimulators. J. South. Orthop. Assoc. 12(2):46–54, 2003.
Ascione, F., A. Vasaturo, S. Caserta, V. D’Esposito, P. Formisano, and S. Guido. Comparison between fibroblast wound healing and cell random migration assays in vitro. Exp. Cell. Res. 347:123–132, 2016.
Ashby, W. J., and A. Zijlstra. Established and novel methods of interrogating two-dimensional cell migration. Integr. Biol. 11:1338–1350, 2012.
Avrahami, R., J. Rosenblum, M. Gazes, and L. Litman. The effect of combined ultrasound and electric field stimulation on wound healing in chronic ulcerations. Wounds 27:199–208, 2014.
Barker, A., L. Jaffe, and J. Vanable. The glabrous epidermis of cavies contains a powerful battery. Am. J. Physiol. 242(3):R358–R366, 1982.
Bourguignon, G. Y., and L. Y. Bourguignon. Electric stimulation of protien and DNA synthesis in human fibroblasts. FASEB J. 1(5):398–402, 1987.
Bourguignon, G. Y., W. Jy, and L. Y. Bourguignon. Electric stimulation of human fibroblasts causes an increase in Ca2+ influx and the exposure of additional insulin receptors. J. Cell Physiol. 140(2):379–385, 1989.
Brown, M. J., and L. M. Loew. Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J. Cell Biol. 127:117–128, 1994.
Calvey, C., W. Zhou, K. S. Stakleff, P. Sendelbach-Sloan, A. B. Harkins, W. Lanzinger, and R. K. Willits. Short-term electrical stimulation to promote nerve repair and functional recovery in a rat model. J. Hand Surg. Am. 40(2):314–322, 2015.
Chao, P., H. Lu, C. Hung, S. Nicoll, and J. Bulinski. Effects of applied DC electric field on ligament fibroblast migration and wound healing. Connect Tissue Res. 48:188–197, 2007.
Collard, J., and M. Hinsenkamp. Cellular processes involved in human epidermal cells exposed to extremely low frequency electric fields. Cell. Signal. 27:889–898, 2015.
Dickinson, R. B., and R. T. Tranquillo. Optimal estimation of cell movement indices from the statistical analysis of cell tracking data. AlChe J. 39(12):1995–2010, 1993.
Erickson, C. A., and R. Nuccitelli. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J. Cell Biol. 98(1):296–307, 1984.
Fang, K. S., B. Farboud, R. Nuccitelli, and R. R. Isseroff. Migration of human keratinocytes in electric fields requires growth factors and extracellular calcium. J. Invest. Dermatol. 11:751–756, 1998.
Farboud, B., R. Nuccitelli, I. R. Schwab, and R. R. Isseroff. DC electric fields induce rapid directional migration in human corneal epithelial cells. Exp. Eye Res. 70:667–673, 2000.
Feedar, J. A., L. C. Kloth, and G. D. Gentzkow. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys. Ther. 71(9):639–649, 1991.
Funari, V. A., M. Winkler, J. Brown, S. D. Dimitrijevic, A. V. Ljubimov, and M. Saghizadeh. Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PLoS ONE 8(12):e84425, 2013.
George, S. P., H. Chen, J. C. Conrad, and S. Khurana. Regulation of directional cell migration by membrane-induced actin binding. J. Cell Sci. 126:312–326, 2013.
Ghosh, K., X. Ren, G. Prestwich, and R. Clark. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 12:601–613, 1006.
Grinnell, F. Fibronectin and wound healing. J. Cell. Biochem. 26(2):107–116, 1984.
Guo, A., B. Song, B. Reid, Y. Gu, J. Forrester, C. Jahoda, and M. Zhao. Effects of physiological electric fields on migration of human dermal fibroblasts. J. Invest. Dermatol. 130:2320–2327, 2010.
Kim, M., M. Lee, B. Kwon, H. Seo, M. Koo, K. You, D. Kim, and J. Park. Control of neonatal human dermal fibroblast migration on poly(lactic-co-glycolic acid)-coated surfaces by electrotaxis. J. Tissue Eng. Regener. Med. 11:11–12, 2015.
Kloth, L. C. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Adv. Wound Care 3(2):81–90, 2014.
Lenselink, E. A. Role of fibronectin in normal wound healing. Int. Wound. J. 12:313–316, 2015.
Lin, F., X. Ren, Z. Pan, L. Macri, W. Zong, M. Tonnesen, M. Rafailovich, D. Bar-Sagi, and R. Clark. Fibronectin growth factor-binding domains are required for fibroblast survival. J. Invest. Dermatol. 131:84–98, 2011.
McCaig, C., A. Rajnicek, B. Song, and M. Zhao. Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 85:943–978, 2005.
McClain, S., M. Simon, E. Jones, A. Nandi, J. Gailit, M. Tonnesen, D. Newman, and R. Clark. Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am. J. Pathol. 149:1257–1270, 1996.
Meijering, E., O. Dzyubachyk, and I. Smal. Methods for cell and particle tracking. Methods Enzymol. 504:183–200, 2012.
Messerli, M., and D. Graham. Extracellular electrical fields direct wound healing and regeneration. Biol. Bull 221(1):79–92, 2011.
Mustoe, T. A., K. O’Shaughnessy, and O. Kloeters. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast. Reconstr. Surg. 117(7 Suppl):35S–41S, 2006.
Natali, P. G., M. R. Nicotra, D. Filippo, and A. Bigotti. Expression of fibronectin, fibronectin isoforms and integrin receptors in melanocytic lesions. Br. J. Cancer 71(6):1243–1247, 1995.
Nishimura, K. Y., R. R. Isseroff, and R. Nuccitelli. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J. Cell Sci. 109:199–207, 1996.
Niu, X., M. Rouabhia, N. Chiffot, M. King, and Z. Zhang. An electrically conductive 3D scaffold based on a nonwoven web of poly(l-lactic acid) and conductive poly(3,4-ethylenedioxythiophene). J. Biomed. Mater. Res. Part A 103(8):2635–2644, 2015.
Park, H., M. Rouabhia, D. Lavertu, and Z. Zhang. Electrical stimulation modulates the expression of multiple wound healing genes in primary human dermal fibroblasts. Tissue Eng. Part A 21(13–14):1982–1990, 2015.
Peters, E. J., L. A. Lavery, D. G. Armstrong, and J. G. Fleischli. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch. Phys. Med. Rehabil. 82(6):721–725, 2001.
Rasband, W. S. ImageJ, 1997–2016. http://imagej.nih.gov/ij/
Rhoads, D. S., and J.-L. Guan. Analysis of directional cell migration on defined FN gradients: role of intracellular signaling molecules. Exp. Cell. Res. 313(18):3859–3867, 2007.
Rouabhia, M., H. Park, S. Meng, H. Derbali, and Z. Zhang. Electrical stimluation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 8(8):e71660, 2013.
Sebastian, A., F. Syeh, D. Perry, V. Balamurugan, J. Colthurst, I. Chaudhry, and A. Bayat. Acceleration of cutaneous healing by electrical stimulation: degeneral electrical waveform down-regulates inflammation, up-regulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Repair Regen. 19(6):693–708, 2011.
Sen, C., G. Gordillo, S. Roy, R. Kirsner, L. Lambert, T. Hunt, F. Gottrup, G. Gurtner, and M. Longaker. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 17(6):763–771, 2009.
Sheridan, D. M., R. R. Isseroff, and R. Nuccitelli. Imposition of a physiologic DC electric field alters the migratory response of human keratinocytes on extracelluar matrix molecules. J Invest. Dermatol. 106(4):642–646, 1996.
Sillman, A. L., D. M. Quang, B. Farboud, K. S. Fang, R. Nuccitelli, and R. R. Isseroff. Human dermal fibroblasts do not exhibit directional migration on collagen I in direct-current electric fields of physiological strength. Exp. Dermatol. 12(4):396–402, 2003.
Simpson, M. J., K. Y. Lo, and Y. S. Sun. Quantifying the roles of random motility and directed motility using advection-diffusion theory for a 3T3 fibroblast cell migration assay stimulated with an electric field. BMC Syst. Biol. 11(1):39, 2017.
Tandon, N., C. Cannizzaro, P. H. Chao, R. Maidhof, A. Marsano, H. T. Au, M. Radisic, and G. Vunjak-Novakovic. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 4(2):155–173, 2009.
Tarantino, N., J. Tinevez, E. F. Crowell, B. Boisson, R. Henriques, M. Mhlanga, F. Agou, A. Israel, and E. Laplantine. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO-KK supramolecular structures. J. Cell Biol. 204(2):231–245, 2014.
Ware, M. F., A. Wells, and D. A. Lauffenburger. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J. Cell Sci. 111(Pt 16):2423–2432, 1998.
Weiss, D., R. Kirsner, and W. Eaglestein. Electrical stimulation and wound healing. Arch. Dermatol. 126(2):222–225, 1990.
Wood, M., and R. K. Willits. Short-duraction, DC electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics 27(4):328–331, 2006.
Zhao, M. Electrical fields in wound healing—an overriding signal that directs cell migration. Semin. Cell Dev. Biol. 20(6):674–682, 2009.
Acknowledgments
Funding for this work was provided through the Margaret F. Donovan Endowed Chair for Women in Engineering at The University of Akron. The authors would like to thank the Cornell University Statistical Consulting Unit for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael Gower oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Snyder, S., DeJulius, C. & Willits, R.K. Electrical Stimulation Increases Random Migration of Human Dermal Fibroblasts. Ann Biomed Eng 45, 2049–2060 (2017). https://doi.org/10.1007/s10439-017-1849-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1849-x