Abstract
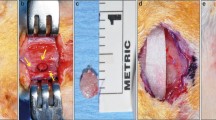
Guided bone regeneration (GBR) procedures using graft materials have been used for reconstruction of osseous defects. The aim of the present in vivo micro-computed tomographic (µCT) and histologic study was to assess in real time the bone regeneration at GBR sites in standardized experimental calvarial defects (diameter 3.3 mm) using β-tricalcium phosphate (β-TCP) with and without collagen membrane (CM). A single full-thickness calvarial defect was created on the left parietal bone in young female Wistar albino rats (n = 30) weighing approximately 300 g and aged about 6 weeks. The animals were randomly divided into three groups for treatment, based on calvarial defect filling material: (1) control group (n = 10); (2) β-TCP + CM group (n = 10); (3) β-TCP group (n = 10). Real-time in vivo µCT analyses were performed immediately after surgery and at 2, 4, 6 and 10 weeks to determine the volume and mineral density of the newly formed bone (BVNFB, MDNFB) and remaining β-TCP particles (VRBP, MDRBP). The animals were killed at 10 weeks and calvarial specimens were evaluated histologically. In the control group, MDNFB increased significantly at 6 weeks (0.32 ± 0.002 g/mm3, P < 0.01) compared to that at baseline. In β-TCP + CM group, BVNFB (1.10 ± 0.12 mm3, P < 0.01) and MDNFB (0.13 ± 0.02 g/mm3, P < 0.01) significantly increased at the 4th week than baseline. In the β-TCP group, BVNFB (1.13 ± 0.12 mm3, P < 0.01) and MDNFB (0.14 ± 0.01 g/mm3, P < 0.01) significantly increased at 6 weeks compared to that at baseline. Significant reduction in VRBP was neither seen in the β-TCP + CM group nor in the β-TCP group. While in the β-TCP + CM group MDRBP was reduced significantly at 6 weeks (0.44 ± 0.9 g/mm3, P < 0.01) from baseline (0.98 ± 0.03 g/mm3), similar significant reduction in MDRBP from baseline (0.92 ± 0.07 g/mm3) was seen only at 10 weeks (0.45 ± 0.06 g/mm3, P < 0.05) in the β-TCP group. Histologic findings at 10 weeks revealed greater amount of NFB with osteocytes in the matrix, in the β-TCP + CM group than in the β-TCP group. Biomechanical assessment of NFB for hardness (H) and elastic modulus (E) revealed significantly higher values for the β-TCP + CM group (H = 612.6 ± 4.28 Mpa; E = 13.57 ± 0.07 Gpa) when compared to those of the control (H = 192.1 ± 4.93 Mpa; E = 6.76 ± 0.04 Gpa) and the β-TCP groups (H = 241.9 ± 6.29 Mpa; E = 4.34 ± 0.06 Gpa). In conclusion, based on real-time assessment, NFB is formed in calvarial defects as early as 4 weeks following GBR with β-TCP + CM as compared to 6 weeks when β-TCP alone was used.







Similar content being viewed by others
References
Hammerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008;19:19–25.
Talreja PS, Gayathri GV, Mehta DS. Treatment of an early failing implant by guided bone regeneration using resorbable collagen membrane and bioactive glass. J Indian Soc Periodontol. 2013;17:131–6.
Koyama Y, Kikuchi M, Edamura K, Nagaoka K, Tanaka S, Tanaka J, et al. Reconstruction of bone fenestration on mandiblar by the guided bone regeneration methods with beta-TCP/PLGC membranes. J Nanosci Nanotechnol. 2007;7:859–61.
Walsh WR, Vizesi F, Michael D, Auld J, Langdown A, Oliver R, et al. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008;29:266–71.
Chopra PM, Johnson M, Nagy TR, Lemons JE. Micro-computed tomographic analysis of bone healing subsequent to graft placement. J Biomed Mater Res B Appl Biomater. 2009;88:611–8.
Zerbo IR, Bronckers AL, de Lange G, Burger EH. Localisation of osteogenic and osteoclastic cells in porous beta-tricalcium phosphate particles used for human maxillary sinus floor elevation. Biomaterials. 2005;26:1445–51.
Ogose A, Kondo N, Umezu H, Hotta T, Kawashima H, Tokunaga K, et al. Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion) in human bones. Biomaterials. 2006;27:1542–9.
Wiltfang J, Merten HA, Schlegel KA, Schultze-Mosgau S, Kloss FR, Rupprecht S, et al. Degradation characteristics of alpha and beta tri-calcium-phosphate (TCP) in minipigs. J Biomed Mater Res. 2002;63:115–21.
Zerbo IR, Zijderveld SA, de Boer A, Bronckers AL, de Lange G, ten Bruggenkate CM, et al. Histomorphometry of human sinus floor augmentation using a porous beta-tricalcium phosphate: a prospective study. Clin Oral Implants Res. 2004;15:724–32.
Ayoub MA, El-Rosasy MA. Hybrid grafting of post-traumatic bone defects using beta-tricalcium phosphate and demineralized bone matrix. Eur J Orthop Surg Traumatol. 2013;24(5):663–70.
Dai LY, Jiang LS. Anterior cervical fusion with interbody cage containing beta-tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-year follow-up. Eur Spine J. 2008;17:698–705.
Epstein NE. Efficacy of posterior cervical fusions utilizing an artificial bone graft expander, beta tricalcium phosphate. Surg Neurol Int. 2011;2:15.
Hirata M, Murata H, Takeshita H, Sakabe T, Tsuji Y, Kubo T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int Orthop. 2006;30:510–3.
Brkovic BM, Prasad HS, Konandreas G, Milan R, Antunovic D, Sandor GK, et al. Simple preservation of a maxillary extraction socket using beta-tricalcium phosphate with type I collagen: preliminary clinical and histomorphometric observations. J Can Dent Assoc. 2008;74:523–8.
Weijs WL, Siebers TJ, Kuijpers-Jagtman AM, Berge SJ, Meijer GJ, Borstlap WA. Early secondary closure of alveolar clefts with mandibular symphyseal bone grafts and beta-tricalcium phosphate (beta-TCP). Int J Oral Maxillofac Surg. 2010;39:424–9.
Kaushick BT, Jayakumar ND, Padmalatha O, Varghese S. Treatment of human periodontal infrabony defects with hydroxyapatite + beta tricalcium phosphate bone graft alone and in combination with platelet rich plasma: a randomized clinical trial. Indian J Dent Res. 2011;22:505–10.
Kishore DT, Bandiwadekar T, Padma R, Debunath S, Profulla, Reddy A. Evaluation of relative efficacy of beta-tricalcium phosphate with and without type I resorbable collagen membrane in periodontal infrabony defects: a clinical and radiographic study. J Contemp Dent Pract. 2013;14:193–201.
Saini N, Sikri P, Gupta H. Evaluation of the relative efficacy of autologous platelet-rich plasma in combination with beta-tricalcium phosphate alloplast versus an alloplast alone in the treatment of human periodontal infrabony defects: a clinical and radiological study. Indian J Dent Res. 2011;22:107–15.
Colangelo P, Piattelli A, Barrucci S, Trisi P, Formisano G, Caiazza S. Bone regeneration guided by resorbable collagen membranes in rabbits: a pilot study. Implant Dent. 1993;2:101–5.
Dahlin C, Sennerby L, Lekholm U, Linde A, Nyman S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989;4:19–25.
Al-Hezaimi K, Salameh Z, Al-Fouzan K, Al Rejaie M, Tay FR. Histomorphometric and micro-computed tomography analysis of pulpal response to three different pulp capping materials. J Endod. 2011;37:507–12.
Kochi G, Sato S, Ebihara H, Hirano J, Arai Y, Ito K. A comparative study of microfocus CT and histomorphometry in the evaluation of bone augmentation in rat calvarium. J Oral Sci. 2010;52:203–11.
Nevins ML, Camelo M, Rebaudi A, Lynch SE, Nevins M. Three-dimensional micro-computed tomographic evaluation of periodontal regeneration: a human report of intrabony defects treated with Bio-Oss collagen. Int J Periodontics Restor Dent. 2005;25:365–73.
Peyrin F. Evaluation of bone scaffolds by micro-CT. Osteoporos Int. 2011;22:2043–8.
Zhao N, Liu Y, Kanzaki H, Liang W, Ni J, Lin J. Effects of local osteoprotegerin gene transfection on orthodontic root resorption during retention: an in vivo micro-CT analysis. Orthod Craniofac Res. 2012;15:10–20.
Dai QG, Zhang P, Wu YQ, Ma XH, Pang J, Jiang LY, et al. Ovariectomy induces osteoporosis in the maxillary alveolar bone: an in vivo micro-CT and histomorphometric analysis in rats. Oral Dis. 2013;20(5):514–20.
Ru N, Liu SS, Zhuang L, Li S, Bai Y. In vivo microcomputed tomography evaluation of rat alveolar bone and root resorption during orthodontic tooth movement. Angle Orthod. 2013;83:402–9.
Beck A, Woods S, Lansdowne JL, Arens D. The effects of multiple high-resolution peripheral quantitative computed tomography scans on bone healing in a rabbit radial bone defect model. Bone. 2013;56:312–9.
Dare A, Hachisu R, Yamaguchi A, Yokose S, Yoshiki S, Okano T. Effects of ionizing radiation on proliferation and differentiation of osteoblast-like cells. J Dent Res. 1997;76:658–64.
Takata T, Wang HL, Miyauchi M. Migration of osteoblastic cells on various guided bone regeneration membranes. Clin Oral Implants Res. 2001;12:332–8.
Wang HL, Carroll MJ. Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int. 2001;32:504–15.
Prathap S, Hegde S, Kashyap R, Prathap MS, Arunkumar MS. Clinical evaluation of porous hydroxyapatite bone graft (Periobone G) with and without collagen membrane (Periocol) in the treatment of bilateral grade II furcation defects in mandibular first permanent molars. J Indian Soc Periodontol. 2013;17:228–34.
Murai M, Sato S, Fukase Y, Yamada Y, Komiyama K, Ito K. Effects of different sizes of beta-tricalcium phosphate particles on bone augmentation within a titanium cap in rabbit calvarium. Dent Mater J. 2006;25:87–96.
Ozdemir B, Okte E. Treatment of intrabony defects with beta-tricalciumphosphate alone and in combination with platelet-rich plasma. J Biomed Mater Res B Appl Biomater. 2012;100:976–83.
Saini A, Singh M, Lal N, Dixit J. Assessment of combination techniques in enhancing the regenerative potential of tricalcium phosphate graft in treatment of infrabony periodontal defects. Indian J Dent Res. 2011;22:391–5.
Divittorio G, Jackson KL, Chindalore VL, Welker W, Walker JB. Examining the relationship between bone mineral density and fracture risk reduction during pharmacologic treatment of osteoporosis. Pharmacotherapy. 2006;26:104–14.
Acknowledgments
The authors would like to acknowledge the “Deanship of Scientific Research” and “College of Dentistry Research Center”(Registration no. FR 0121), King Saud University, Riyadh, Saudi Arabia for their help and support.
Conflict of interest
The authors declare that they have no conflicts of interest whatsoever.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramalingam, S., Al-Rasheed, A., ArRejaie, A. et al. Guided bone regeneration in standardized calvarial defects using beta-tricalcium phosphate and collagen membrane: a real-time in vivo micro-computed tomographic experiment in rats. Odontology 104, 199–210 (2016). https://doi.org/10.1007/s10266-015-0211-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-015-0211-8