Abstract
Background
Dendritic cell (DC)-based and cytokine-induced killer cell (CIK)-based therapy can induce specific antitumor T-cell responses. This clinical pilot study examined the safety, the feasibility, and the outcome of tumor-specific immunotherapy for patients with advanced pancreatic adenocarcinoma.
Methods
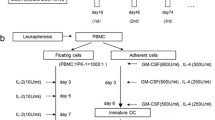
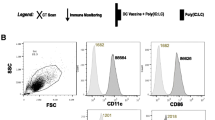
Alpha-Gal epitopes were synthesised on pancreatic carcinoma cell membranes with α1,3-galactosyltransferase in vitro. Subsequently, the addition of natural human anti-Gal IgG to the processed membranes resulted in opsonization and effective phagocytosis by DCs, which were co-cultured with newly differentiated CIKs from bone marrow stem cells to generate tumor-specific immune responders ex vivo. Fourteen patients with inoperable stage III/IV pancreatic adenocarcinoma were enrolled in the study; the treatment procedure consisted of injections of DCs and CIKs.
Results
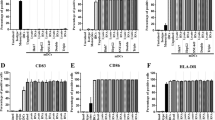
Clinical observation showed that the procedure was safe and lacked serious side effects. Tests showed that 12 patients had strong positive delayed-type IV hypersensitivity to the autologous cancer cell lysate; robust systemic cytotoxicity elicited by interferon (IFN)γ expression by peripheral blood mononuclear cells; and significant increases in CD3+CD8+, CD3+CD45RO+, and CD3+CD56+ cells in peripheral blood lymphocytes after 3 injections. During the follow up, the percentages of CD3+CD8+, CD3+CD45RO+, and CD3+CD56+ cells returned to the normal range at 6 to 9 months after the third injection and IFNγ expression in the cells stayed at the higher level from the third injection to 24 months after the treatment.
Conclusions
This new tumor-specific immunotherapy is safe, feasible, and has great potential for pancreatic carcinoma treatment.



Similar content being viewed by others
References
De Moor V, Arvanitakis M, Nagy N et al (2012) Intraductal papillary mucinous neoplasms of the pancreas: clinicopathological features and long term outcome related to histopathological group. Hepatogastroenterology 59:565–569
Neuzillet C, Sauvanet A, Hammel P (2011) Prognostic factors for resectable pancreatic adenocarcinoma. J Visc Surg 148:e232–e243
Bellone G, Novarino A, Vizio B et al (2009) Impact of surgery and chemotherapy on cellular immunity in pancreatic carcinoma patients in view of an integration of standard cancer treatment with immunotherapy. Int J Oncol 34:1701–1715
Hance KW, Rogers CJ, Zaharoff DA et al (2009) The antitumor and immunoadjuvant effects of IFN-alpha in combination with recombinant poxvirus vaccines. Clin Cancer Res 15:2387–2396
Fogar P, Basso D, Fadi E et al (2011) Pancreatic cancer alters human CD4+ T lymphocyte function: a piece in the immune evasion puzzle. Pancreas 40:1131–1137
Bauer C, Dauer M, Saraj S et al (2011) Dendritic cell-based vaccination of patients with advanced pancreatic carcinoma: results of a pilot study. Cancer Immunol Immunother 60:1097–1107
Mule JJ (2009) Dendritic cell-based vaccines for pancreatic cancer and melanoma. Ann N Y Acad Sci 1174:33–40
Van Gool S (2009) The dendritic therapy with its potential applications in pancreatic cancer. Acta Gastroenterol Belg 72:338–343
Shi M, Zhang B, Tang ZR et al (2004) Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol 10:1146–1151
Chauvin C, Josien R (2008) Dendritic cells as killers: mechanistic aspects and potential roles. J Immunol 181:11–16
Deguchi T, Tanemura M, Miyoshi E et al (2010) Increased immunogenicity of tumor-associated antigen, mucin 1, engineered to express alpha-gal epitopes: a novel approach to immunotherapy in pancreatic cancer. Cancer Res 70:5259–5269
Galili U, Chen ZC, DeGeest K (2003) Expression of alpha-gal epitopes on ovarian carcinoma membranes to be used as a novel autologous tumor vaccine. Gynecol Oncol 90:100–108
Poirier N, Blancho G (2008) Recombinant human C1-inhibitor inhibits cytotoxicity induced by allo- and xenoantibodies. Transpl Proc 40:581–583
Buonomano R, Tinguely C, Rieben R et al (1999) Quantitation and characterization of anti-Galalpha1-3Gal antibodies in sera of 200 healthy persons. Xenotransplantation 6:173–180
Galili U (2005) The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol 83:674–686
Knutson KL, Disis ML (2005) Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 54:721–728
Krishnan S, Rana V, Janjan NA et al (2006) Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer 107:2589–2596
Macher BA, Anaraki F et al (1994) Defining the minimal size of catalytically active primate alpha-1,3-galactosyltransferase: structure-function studies on the recombinant truncated enzyme. Glycobiology 4:193–201
Adamson L, Palmborg A, Svensson A et al (2004) Development of a technology platform for large-scale clinical grade production of DC. Cytotherapy 6:363–371
Wang QJ, Wang H, Pan K et al (2010) Comparative study on anti-tumor immune response of autologous cytokine-induced killer (CIK) cells, dendritic cells-CIK (DC-CIK), and semi-allogeneic DC-CIK. Chin J Cancer 29:641–648
Rezai P, Mulcahy MF, Tochetto SM et al (2009) Morphological analysis of pancreatic adenocarcinoma on multidetector row computed tomography: implications for treatment response evaluation. Pancreas 38:799–803
Kalish RS, Askenase PW (1999) Molecular mechanisms of CD8+ T cell-mediated delayed hypersensitivity: implications for allergies, asthma, and autoimmunity. J Allergy Clin Immunol 103(2 Pt 1):192–199
Lee KH, Kim MK, Kim YH et al (2009) Gemcitabine and oxaliplatin combination as first-line treatment for advanced pancreatic cancer: a multicenter phase II study. Cancer Chemother Pharmacol 64:317–325
Acknowledgments
This work was supported by the Hong Kong Wang Kuan Cheng Grant and Inner Mongolia Stem Cell Grant (Grant code: kjk10jhg). We also thank Professor Jin Gao, who processed the α-1,3-Gal epitope-pulsed DCs and CIKs at the Jing Meng Stem Cell Company, Beijing, China.
Conflict of interest
All authors declared that they had no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Qiu, Y., Yun, M.M., Xu, M.B. et al. Pancreatic carcinoma-specific immunotherapy using synthesised alpha-galactosyl epitope-activated immune responders: findings from a pilot study. Int J Clin Oncol 18, 657–665 (2013). https://doi.org/10.1007/s10147-012-0434-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0434-4