Abstract
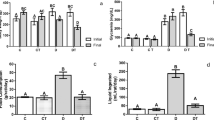
The aim of the present study was to analyze the effect of low-power laser irradiation in the antioxidant enzymatic system of submandibular (SMG) and parotid (PG) salivary glands of streptozotocin-induced diabetic rats. The animals were randomly divided into six groups: three diabetic groups (D0, D5, and D20) and three non-diabetic groups (C0, C5, and C20), according to laser dose received (0, 5, and 20 J/cm2, respectively). Areas of approximately 1 cm2 were demarcated in the salivary glands (each parotid and both submandibular glands) and after irradiated according to Simões et.al. (Lasers Med Sci 24:202–208, 2009). A diode laser (660 nm/100 mW) was used, with laser beam spot of 0.0177 cm2. The group treated with 5 J/cm2 laser dose was subjected to irradiation for 1 min and 4 s (total irradiation time) and the group treated with 20 J/cm2 laser dose was subjected to irradiation for 4 min and 16 s. Twenty-four hours after irradiation the animals were euthanized and the salivary glands were removed for biochemical analysis. The total antioxidant values (TA), the activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase enzymes were determined. SOD and CAT activities, as well as TA were higher in SMG of irradiated diabetic rats. However, in SMG of non-diabetic rats, laser irradiation decreased TA values and led to an increase in the CAT activity. In addition, there was a decrease in the activity of CAT in PG of diabetic and non-diabetic animals after laser irradiation. According to the results of the present study, low-power laser irradiation can affect the enzymatic antioxidant system of salivary glands of streptozotocin-induced diabetic rats.



Similar content being viewed by others
References
Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003 26 Suppl 1:S5–20
Darnell JA, Saunders MJ (1990) Oral manifestations of the diabetic patient. Tex Dent J 107(2):23–27
Loe H (1993) Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16(1):329–334
Ship JA (2003) Diabetes and oral health: an overview. J Am Dent Assoc 134 Spec No:4 S–10S
Mata AD, Marques D, Rocha S, Francisco H, Santos C, Mesquita MF et al (2004) Effects of diabetes mellitus on salivary secretion and its composition in the human. Mol Cell Biochem 261(1–2):137–142
Moore PA, Guggenheimer J, Etzel KR, Weyant RJ, Orchard T (2001) Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92(3):281–291
Dodds MW, Yeh CK, Johnson DA (2000) Salivary alterations in type 2 (non-insulin-dependent) diabetes mellitus and hypertension. Community Dent Oral Epidemiol 28(5):373–381
Yoon MS, Jankowski V, Montag S, Zidek W, Henning L, Schluter H et al (2004) Characterisation of advanced glycation endproducts in saliva from patients with diabetes mellitus. Biochem Biophys Res Commun 323(2):377–381
Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF (2000) Type 2 diabetes and oral health: a comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract 50(1):27–34
Ben-Aryeh H, Serouya R, Kanter Y, Szargel R, Laufer D (1993) Oral health and salivary composition in diabetic patients. J Diabetes Complications 7(1):57–62
Oberley LW (1988) Free radicals and diabetes. Free Radic Biol Med 5(2):113–124
Nogueira FN, Carvalho AM, Yamaguti PM, Nicolau J (2005) Antioxidant parameters and lipid peroxidation in salivary glands of streptozotocin-induced diabetic rats. Clin Chim Acta 353(1–2):133–139
Turner S, Zettler G, Arcos ML, Cremaschi G, Davicino R, Anesini C (2011) Effect of streptozotocin on reactive oxygen species and antioxidant enzyme secretion in rat submandibulary glands: a direct and an indirect relationship between enzyme activation and expression. Eur J Pharmacol 659(2–3):281–288
Halliwell B, Gutteridge, JMC, editor. Free radicals in biology and medicine. 4th edition ed. Oxford, New York: Oxford University Press 2007
Ibuki FK, Simoes A, Nogueira FN (2010) Antioxidant enzymatic defense in salivary glands of streptozotocin-induced diabetic rats: a temporal study. Cell Biochem Funct 28(6):503–508
Nicolau J, de Matos JA, de Souza DN, Neves LB, Lopes AC (2005) Altered glycogen metabolism in the submandibular and parotid salivary glands of rats with streptozotocin-induced diabetes. J Oral Sci 47(2):111–116
Nicolau J, de Souza DN, Carrilho M (2009) Increased glycated calmodulin in the submandibular salivary glands of streptozotocin-induced diabetic rats. Cell Biochem Funct 27(4):193–198
Nicolau J, De Souza DN, Simoes A (2009) Alteration of Ca(2+)-ATPase activity in the homogenate, plasma membrane and microsomes of the salivary glands of streptozotocin-induced diabetic rats. Cell Biochem Funct 27(3):128–134
Simoes A, Ganzerla E, Yamaguti PM, de Paula Eduardo C, Nicolau J (2009) Effect of diode laser on enzymatic activity of parotid glands of diabetic rats. Lasers Med Sci 24(4):591–596
Simoes A, Nicolau J, de Souza DN, Ferreira LS, de Paula Eduardo C, Apel C et al (2008) Effect of defocused infrared diode laser on salivary flow rate and some salivary parameters of rats. Clin Oral Investig 12(1):25–30
Simoes A (2010) Nogueira FN, de Paula Eduardo C. Nicolau J Diode laser decreases the activity of catalase on submandibular glands of diabetic rats Photomed Laser Surg 28(1):91–95
Simoes A, Platero MD, Campos L, Aranha AC, Eduardo Cde P, Nicolau J (2009) Laser as a therapy for dry mouth symptoms in a patient with Sjogren's syndrome: a case report. Spec Care Dent 29(3):134–137
Simoes A, Siqueira WL, Lamers ML, Santos MF, Eduardo Cde P, Nicolau J (2009) Laser phototherapy effect on protein metabolism parameters of rat salivary glands. Lasers Med Sci 24(2):202–208
Fahimipour F, Mahdian M, Houshmand B, Asnaashari M, Sadrabadi AN, Farashah SE, et al (2012) The effect of He–Ne and Ga–Al–As laser light on the healing of hard palate mucosa of mice. Lasers Med Sci
Nouruzian M, Alidoust M, Bayat M, Akbari M (2012) Effect of low-level laser therapy on healing of tenotomized Achilles tendon in streptozotocin-induced diabetic rats. Lasers Med Sci
Tunér J, Hode L, editor. Low level laser therapy—clinical practice and scientific background. Prima Book. Grängesberg, Sweden 1999
Kats AG, Belostotskaia IM, Malomud ZP, Makarova LG (1985) Remote results of the complex treatment of chronic sialadenitis using the helium–neon laser. Vestn Khir Im I I Grek 135(10):39–42
Jung KaH W (1996) Developmental changes of antioxidants enzymes in kidney and liver from rats. Free Radic Biol Med 20(4):613–617
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 84(4):407–412
Suttle NF, McMurray CH (1983) Use of erythrocyte copper:zinc superoxide dismutase activity and hair or fleece copper concentrations in the diagnosis of hypocuprosis in ruminants. Res Vet Sci 35(1):47–52
Woolliams JA, Wiener G, Anderson PH, McMurray CH (1983) Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci 34(3):253–256
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Aebi H, editor. Catalase in vitro 1984
Simoes A, de Oliveira E, Campos L, Nicolau J (2009) Ionic and histological studies of salivary glands in rats with diabetes and their glycemic state after laser irradiation. Photomed Laser Surg 27(6):877–883
Patel DG, Begum N, Smith PH (1986) Insulin-like material in parotid and submaxillary salivary glands of normal and diabetic adult male mice. Diabetes 35(7):753–758
Kerr M, Lee A, Wang PL, Purushotham KR, Chegini N, Yamamoto H et al (1995) Detection of insulin and insulin-like growth factors I and II in saliva and potential synthesis in the salivary glands of mice. Effects of type 1 diabetes mellitus. Biochem Pharmacol 49(10):1521–1531
Anderson LC (1983) Effects of alloxan diabetes and insulin in vivo on rat parotid gland. Am J Physiol 245(3):G431–G437
Wang D, Yuan Z, Inoue N, Cho G, Shono M, Ishikawa Y (2011) Abnormal subcellular localization of AQP5 and downregulated AQP5 protein in parotid glands of streptozotocin-induced diabetic rats. Biochim Biophys Acta 1810(5):543–554
Mahay S, Adeghate E, Lindley MZ, Rolph CE, Singh J (2004) Streptozotocin-induced type 1 diabetes mellitus alters the morphology, secretory function and acyl lipid contents in the isolated rat parotid salivary gland. Mol Cell Biochem 261(1–2):175–181
Watanabe M, Yamagishi-Wang H, Kawaguchi M (2001) Lowered susceptibility of muscarinic receptor involved in salivary secretion of streptozotocin-induced diabetic rats. Jpn J Pharmacol 87(2):117–124
Kakkar R, Kalra J, Mantha SV, Prasad K (1995) Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 151(2):113–119
Gumieniczek A, Hopkala H, Wojtowicz Z, Nieradko M (2001) Differences in antioxidant status in skeletal muscle tissue in experimental diabetes. Clin Chim Acta 314(1–2):39–45
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Onizawa K, Muramatsu T, Matsuki M, Ohta K, Matsuzaka K, Oda Y et al (2009) Low-level (gallium–aluminum–arsenide) laser irradiation of Par-C10 cells and acinar cells of rat parotid gland. Laser Med Sci 24(2):155–161
Tuner J, Hode L (1998) It's all in the parameters: a critical analysis of some well-known negative studies on low-level laser therapy. J Clin Laser Med Surg 16(5):245–248
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56(5):691–704
Nicolau J, Sassaki KT (1976) Metabolism of carbohydrate in the major salivary glands of rats. Arch Oral Biol 21(11):659–661
Schenkels LC, Veerman EC, Nieuw Amerongen AV (1995) Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med 6(2):161–175
Kirkman HN, Gaetani GF (2007) Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci 32(1):44–50
Artyukhov VG, Basharina OV, Pantak AA, Sveklo LS (2000) Effect of helium–neon laser on activity and optical properties of catalase. Bull Exp Biol Med 129(6):537–540
Acknowledgments
The authors wish to express their gratitude to the Special Laboratory of Lasers in Dentistry (LELO) and to FAPESP (Fundação de Apoio a Pesquisa do Estado de São Paulo, #08/009787-5 and #2011/14013-1), for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibuki, F.K., Simões, A., Nicolau, J. et al. Laser irradiation affects enzymatic antioxidant system of streptozotocin-induced diabetic rats. Lasers Med Sci 28, 911–918 (2013). https://doi.org/10.1007/s10103-012-1173-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1173-5