Abstract
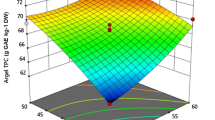
Ultrasound-assisted extraction (UAE) was optimized for extraction of flavonoids and phenolics from dried leaves of Ocimum tenuiflorum using response surface methodology with a central composite rotatable design and independent variables of ethanol concentration (30-70%), sonication time (5-15 min), and ultrasonic energy density (UED) (0.15-0.35 W/cm3). A significant (p<0.0001) quadratic model described experimental data with a non-significant lack of fit (p>0.05). Linear and quadratic effects of independent variables significantly (p<0.001) influenced flavonoid content (FC) and total phenolic content (TPC) yields. Optimized UAE conditions of ethanol concentration, sonication time, and UED were 55.34%, 11.71 min, and 0.26 W/cm3, respectively, resulting in predicted FC and TPC yields of 6.69 mg of quercetin equivalents QE/g and 9.41 mg of gallic acid equivalents GAE/g, respectively. HPLC analysis revealed different phenolic and flavonoid compounds. UAE extracts exhibited better in vitro antioxidant activities and thermo-oxidative stability values than conventional solvent extracts.
Similar content being viewed by others
References
Simon JE, Morales MR, Phippen WB, Vieira RF, Hao Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. pp. 499–505. In: Perspectives on New Crops and New Uses. Janick J (ed). American Society for Horticultural Science Press, Alexandria, VA, USA (1999)
Nakatani N. Antioxidants from spices and herbs. pp. 64–75. In: Natural Antioxidants: Chemistry, Health Effects, and Applications. Shahidi F(ed). AOAC Press, Champaign, IL, USA (1997)
Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. 42: 129–133 (2003)
Upadhyay R, Ramalakshmi K, Rao LJM. Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chem. 130: 184–188 (2012)
Chemat F, Huma Z, Khan MK. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 18: 813–835 (2011)
Pingret D, Fabiano-Tixier AS, Bourvellec CL, Renard CMGC, Chemat F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 111: 73–81 (2012)
Aybastier O, Isik E, Sahin S, Demir C. Optimization of ultrasonic-assisted extraction of antioxidant compounds from blackberry leaves using response surface methodology. Ind. Crop. Prod. 44: 558–565 (2013)
Pan G, Yu G, Zhu C, Qiao J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason. Sonochem. 19: 486–490 (2012)
Hossain MB, Brunton NP, Patras A, Tiwari B, O’Donnell CP, Martin-Diana AB, Barry-Ryan C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram using response surface methodology. Ultrason. Sonochem. 19: 582–590 (2012)
Xia EQ, Ai XX, Zang SY, Guan TT, Xu XR, Li HB. Ultrasound-assisted extraction of phillyrin from Forsythia suspense. Ultrason. Sonochem. 18: 549–552 (2011)
Mason TJ, Lorimer JP, Bates DM. Quantifying sonochemistry: Casting some light on a ‘black art’. Ultrasonics 30: 40–42 (1992)
Upadhyay R, Jha A, Singh SP, Kumar A, Singh M. Appropriate solvents for extracting total phenolics, flavonoids and ascorbic acid from different kinds of millets. J. Food Sci. Tech.-Mys. 52: 472–478 (2013)
Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 299: 152–178 (1999)
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28: 25–30 (1995)
Popov I, Lewin G. Antioxidative homeostasis: Characterization by means of chemiluminescent technique in methods in enzymology. pp. 96–100. In: Oxidants and Antioxidants. Packer L (ed). Academic Press, Waltham, MA, USA (2009)
Upadhyay R, Mishra HN. Antioxidant activity measurement of oleoresin from rosemary and sage. Ind. Crop. Prod. 61: 453–459 (2014)
Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 44: 307–315 (1986)
Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 315: 161–169 (1994)
Wang H, Helliwell K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res. Int. 34: 223–227 (2001)
Jerman T, Trebše P, Mozetiè Vodopivec B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 123: 175–182 (2010)
Dey S, Rathod VK. Ultrasound aßsisted extraction of ß-carotene from Spirulina platensis. Ultrason. Sonochem. 20: 271–276 (2013)
Pingret D, Fabiano-Tixier AS, Chemat F. Degradation during application of ultrasound in food processing: A review. Food Control 31: 593–606 (2013)
Tiwari BK, ODonnell CP, Patras A, Cullen PJ. Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. J. Agr. Food Chem. 56: 10071–10077 (2008)
Rawson A, Tiwari BK, Patras A, Brunton N, Brennan C, Cullen PJ, O’Donnell C. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 44: 1168–1173 (2011)
Dahmoune F, Boulekbache L, Moussi K, Aoun O, Spigno G, Madani K. Valorization of Citrus limon r esidues for t he r ecovery of a ntioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crop. Prod. 50: 77–87 (2013)
Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of tulsi, Ocimum sanctum Linn, as a medicinal plant. Indian J. Exp. Biol. 40: 765–773 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Upadhyay, R., Nachiappan, G. & Mishra, H.N. Ultrasound-assisted extraction of flavonoids and phenolic compounds from Ocimum tenuiflorum leaves. Food Sci Biotechnol 24, 1951–1958 (2015). https://doi.org/10.1007/s10068-015-0257-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-015-0257-y