Abstract
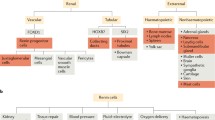
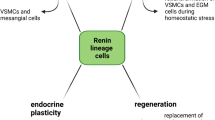
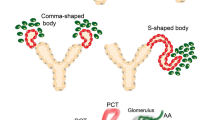
Renin-expressing cells appear early in the embryo and are distributed broadly throughout the body as organogenesis ensues. Their appearance in the metanephric kidney is a relatively late event in comparison with other organs such as the fetal adrenal gland. The functions of renin cells in extra renal tissues remain to be investigated. In the kidney, they participate locally in the assembly and branching of the renal arterial tree and later in the endocrine control of blood pressure and fluid-electrolyte homeostasis. Interestingly, this endocrine function is accomplished by the remarkable plasticity of renin cell descendants along the kidney arterioles and glomeruli which are capable of reacquiring the renin phenotype in response to physiological demands, increasing circulating renin and maintaining homeostasis. Given that renin cells are sensors of the status of the extracellular fluid and perfusion pressure, several signaling mechanisms (β-adrenergic receptors, Notch pathway, gap junctions and the renal baroreceptor) must be coordinated to ensure the maintenance of renin phenotype—and ultimately the availability of renin—during basal conditions and in response to homeostatic threats. Notably, key transcriptional (Creb/CBP/p300, RBP-J) and posttranscriptional (miR-330, miR125b-5p) effectors of those signaling pathways are prominent in the regulation of renin cell identity. The next challenge, it seems, would be to understand how those factors coordinate their efforts to control the endocrine and contractile phenotypes of the myoepithelioid granulated renin-expressing cell.

Similar content being viewed by others
References
Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA (2001) Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281:F345–F356
Sequeira Lopez ML, Gomez RA (2011) Development of the renal arterioles. J Am Soc Nephrol 22:2156–2165
Nishimura H, Ogawa M, Sawyer WH (1973) Renin-angiotensin system in primitive bony fishes and a holocephalian. Am J Physiol 224:950–956
Taugner R, Hackenthal E (1989) The juxtaglomerular apparatus: structure and function. Springer, Berlin Heidelberg New York, pp 104–126
Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ (1989) Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 257:F850–F858
Ice KS, Geary KM, Gomez RA, Johns DW, Peach MJ, Carey RM (1988) Cell and molecular studies of renin secretion. Clin Exp Hypertens A 10:1169–1187
Keeton TK, Campbell WB (1980) The pharmacologic alteration of renin release. Pharmacol Rev 32:81–227
Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA (2004) Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6:719–728
Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM (1990) Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol 259:F660–F665
Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM (1988) Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol 254:F900–F906
Gomez RA, Norwood VF (1995) Developmental consequences of the renin–angiotensin system. Am J Kidney Dis 26:409–431
Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O (1999) Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem 274:14210–14217
Tufro-McReddie A, Arrizurieta EE, Brocca S, Gomez RA (1992) Dietary protein modulates intrarenal distribution of renin and its mRNA during development. Am J Physiol 263:F427–F435
Tufro-McReddie A, Chevalier RL, Everett AD, Gomez RA (1993) Decreased perfusion pressure modulates renin and ANG II type 1 receptor gene expression in the rat kidney. Am J Physiol 264:R696–R702
Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez MLS (2013) Pericytes synthesize renin. World J Nephrol 2:11–16
Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA (2011) Genes that confer the identity of the renin cell. J Am Soc Nephrol 22:2213–2225
Song L, Tuan RS (2006) MicroRNAs and cell differentiation in mammalian development. Birth Defects Res C Embryo Today 78:140–149
Stefani G, Slack FJ (2008) Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9:219–230
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S (2005) A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA 102:16426–16431
Medrano S, Monteagudo MC, Sequeira-Lopez MLS, Pentz ES, Gomez RA (2011) Two microRNAs -miR-330 and miR-125b-5p- mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am J Physiol Renal Physiol 302(1):F29–F37
Persson PB, Skalweit A, Mrowka R, Thiele BJ (2003) Control of renin synthesis. Am J Physiol Regul Integr Comp Physiol 285:R491–R497
Everett AD, Carey RM, Chevalier RL, Peach MJ, Gomez RA (1990) Renin release and gene expression in intact rat kidney microvessels and single cells. J Clin Invest 86:169–175
Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, Kane C, Sigmund CD, Gross KW (2001) Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem 276:45530–45538
Todorov VT, Volkl S, Friedrich J, Kunz-Schughart LA, Hehlgans T, Vermeulen L, Haegeman G, Schmitz ML, Kurtz A (2005) Role of CREB1 and NF{kappa}B-p65 in the down-regulation of renin gene expression by tumor necrosis factor {alpha}. J Biol Chem 280:24356–24362
Klar J, Sandner P, Muller MW, Kurtz A (2002) Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch 444:335–344
Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J (2007) Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol 292:F27–F37
Chen L, Faulhaber-Walter R, Wen Y, Huang Y, Mizel D, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J (2010) Renal failure in mice with Gsalpha deletion in juxtaglomerular cells. Am J Nephrol 32:83–94
Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez ML, Gomez RA (2011) Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics 43:1021–1028
Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de WC, Kurtz A (2010) Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int 78:762–768
Takahashi N, Lopez ML, Cowhig JE Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O (2005) Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16:125–132
Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A (2000) Renin-dependent cardiovascular functions and renin-independent blood–brain barrier functions revealed by renin-deficient mice. J Biol Chem 275:5–8
Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O (1995) Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92:2735–2739
Kihara M, Umemura S, Sumida Y, Yokoyama N, Yabana M, Nyui N, Tamura K, Murakami K, Fukamizu A, Ishii M (1998) Genetic deficiency of angiotensinogen produces an impaired urine concentrating ability in mice. Kidney Int 53:548–555
Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T (1995) Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest 96:2947–2954
Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA (1997) Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension 29:216–221
Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O’Brien DA, Smithies O (1995) Male–female differences in fertility and blood pressure in ACE-deficient mice. Nature 375:146–148
Esther CR Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE (1996) Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Investig 74:953–965
Inokuchi S, Kimura K, Sugaya T, Inokuchi K, Murakami K, Sakai T (2001) Hyperplastic vascular smooth muscle cells of the intrarenal arteries in angiotensin II type 1a receptor null mutant mice. Kidney Int 60:722–731
Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM (1998) Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA 95:15496–15501
Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I (1998) Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest 101:755–760
Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA (2004) Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol 286:R474–R483
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomez, R.A., Belyea, B., Medrano, S. et al. Fate and plasticity of renin precursors in development and disease. Pediatr Nephrol 29, 721–726 (2014). https://doi.org/10.1007/s00467-013-2688-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2688-0