Abstract
Antigen 85B (Ag85B) is an important immunodominant antigen of Mycobacterium tuberculosis, and is a very promising vaccine candidate molecule. Rv3425 is a member of the subgroup 3 of the PPE family, which does not exist in all BCG strains. In this study we constructed a new rBCG which included this united gene (Ag85B-Rv3425). The level of antigen-stimulated T cells expressing IFN-γ was significantly higher in the C57BL/6 mice vaccinated with rBCG::Ag85B-Rv3425 than with BCG. In addition, the sera from mice immunized with rBCG::Ag85B-Rv3425 revealed an increase in the specific immunoglobulin G titers than that from mice immunized with BCG. Antigen specific IgG subclass analysis showed that rBCG::Ag85B-Rv3425 tended to facilitate IgG2a production, suggesting enhancement of predominant Th1 response which in turn may facilitate increased production of protective IFN-γ. These results suggested that this rBCG::Ag85B-Rv3425 could be a strong vaccine candidate for further study.
Similar content being viewed by others
Introduction
The only available TB vaccine, bacillus Calmette–Guérin (BCG), has been distributed since the 1920s and more than three billion people have received this vaccine [1]. However, even today the safety aspects, loss of sensitivity to tuberculin as a diagnostic reagent, and varying efficacy in different trials of BCG have been the subject of extensive debate. Apparently, BCG vaccine used for 80 years, has failed to control TB epidemic. Therefore, there is an urgent need to develop better or improved TB vaccines as an alternative to BCG.
Deciphering of the total Mycobacterium tuberculosis genome sequences [2] and the general advances in comparative genomics have made it possible to design refined BCG vaccines with insertion of genes lost during attenuation of the strain for vaccine use [3]. An effective vaccine requires the ability to elicit protective immune response. The Th1-type immune response is believed to be necessary for protection against mycobacterial pathogens, such as M. tuberculosis and M. bovis [4]. The finding of a new immunodominant antigen may contribute to the development of improved vaccine. Data from humans and several animal models have suggested that T-helper type 1 (Th1) subset and IFN-γ are involved in the development of protective immunity against M. tuberculosis. Thus, increase in Th1 responses or induction higher levels of IFN-γ should lead to increased anti-mycobacterial activity. For development of an improved BCG vaccine, it has also been suggested that over-expression of immunodominant antigens in BCG can also lead to improved induction of Th1 responses [5].
Secreted proteins of M. tuberculosis are recognized early in the course of experimental TB infection of mice and by T cells of TB patients; therefore, they are considered preferential antigenic targets for vaccine development [6, 7]. The antigen 85 complex (Ag85A, B, C) are expressed by nearly all mycobacterial species analyzed so far [8]. It was reported that immunization of mice with rBCG over-expressing Ag85A elicited specific helper responses with a Th1-like phenotype, while the rBCG::Ag85C strain was not effective with respect to IFN-γ production [9]. However, immunization of guinea pigs with antigen 85B preparation together with adjuvant induced protective immunity against TB [10, 11].
The ORF Rv3425 selected for the present study is a member of the subgroup 3 of the PPE family and is located in RD11 [12, 13], which does not exist in all BCG strains. Our lab’s previous study demonstrated that the PPE protein Rv3425 was expressed in M. tuberculosis H37Rv during exponential growth in vitro, and recognized by both pulmonary TB and extra-pulmonary TB patients [14]. We also have evaluated the immunogenicity of Rv3425 protein in mice, and the Rv3425 protein was proved to induce high levels of IFN-γ in mice immunized with the Rv3425 antigen preparation together with adjuvant (submitted for publication).
Reengineering BCG is an interesting approach, and it retains many advantages, such as sharing the remarkable safety, low side effects of BCG, inexpensive and easy to mass produce [15]. Recombinant BCG relies on the basic premise that the efficacy of BCG could be enhanced through insertion of genes encoding immunodominant antigens or immunostimulatory cytokines. The first rBCG vaccine reported to induce greater protective immunity to TB than the standard BCG vaccine in animal models was rBCG30, a BCG Tice strain over-expressing the 30 kDa major secretory protein Ag85B [16]. This vaccine is in Phase I trial in the USA. Another rBCG that expresses two epitopes from ESAT-6 has more recently been constructed [17, 18].
In the present study, a recombinant BCG over-expressing Ag85B-Rv3425 fusion protein was developed, and its ability to elicit antigen-specific immune response in C57BL/6 mice was investigated.
Materials and methods
Bacterial strains and cultures
Mycobacterium bovis BCG Danish strain (obtained from Shanghai Biological Products Institute), rBCG, and M. tuberculosis strain H37Rv were grown on Middlebrook 7H9 medium (Difco Laboratories, Detroit, Michigan, USA) supplemented with 0.5% glycerol, 0.05% Tween 80 and 10% ADC or on solid Middlebrook 7H10 medium (Difco Laboratories) supplemented with OADC or on Sauton medium containing 0.25 g of MgSO4·7H2O, 0.25 g of K2HPO4, 1 g of citric acid, 4 g of sodium glutamate, 30 ml of glycerol, 5 mg of ZnSO4, and 25 mg of ferrum-ammonium citrate in 500 ml. When required, the antibiotic kanamycin (Km) was added at a concentration of 25 μg/ml. Escherichia coli DH5α was grown in Luria-Bertani medium and used for cloning.
Construction of a recombinant BCG containing Ag85B and Rv3425
A recombinant M. bovis BCG substrain Danish was produced by transfection of BCG-Danish strain cells with pMV261-Ag85B-Rv3425. Coding sequences for Ag85B (containing signal sequence) and Rv3425 were amplified from the M. tuberculosis H37Rv genomic DNA, respectively, by PCR using the primers shown in Table 1. The Ag85B and Rv3425-coding regions were cloned into the mycobacterial–E. coli shuttle vector pMV261, in which gene expression is under the control of the strong M. bovis HSP60 promoter. Inserted genes were sequencing confirmed. BCG was transformed with the recombinant plasmid by electroporation as described previously [19] and selected on Middlebrook 7H10 agar (BBL Microbiology Systems, Cockeyville, MD) containing 10% OADC enrichment (BBL Microbiology Systems) and 25 μg/ml kanamycin. The resulting recombinant clones containing either pMV261-Ag85B-Rv3425 or pMV261 were designated rBCG::Ag85B-Rv3425 and rBCG::pMV261, respectively.
Western blot detection of expressed Ag85B and Rv3425
Expression of the recombinant antigens by rBCG::Ag85B-Rv3425 was determined by Western blot. The transformed BCG cells were plated on 7H10 medium supplemented with 25 μg/ml kanamycin and grown at 37°C for 3 weeks, individual colonies were picked and grown in Sauton medium containing 25 μg/ml of kanamycin. After 2 weeks growth, protein expression was induced by heating at 45°C for 60 min. The bacterial cells were centrifuged at 8,000 × g for 20 min. The culture supernatants were concentrated as described previously [20]. 12 μg of the concentrated culture filtrates from rBCG::Ag85B-Rv3425 were analyzed for expression of recombinant antigens by western blot using anti-Ag85B and anti-Rv3425 rabbit polyclonal anti-sera, respectively.
Animals and immunization
Female C57BL/6 mice of Slaccas Inc. (Shanghai, China), aging 5–6 weeks each, were used in a P2-level animal facility at Second Military Medical University, Shanghai, China. Mice received free access to food and water throughout this study. All experiments were performed in accordance to the local ethics committee. For analysis of bacterial persistence, C57BL/6 mice (sixteen per group) were infected via the lateral tail vein (intravenous; i.v.) with 5 × 106 CFU of BCG or rBCG::Ag85B-Rv3425. Bacterial persistence and dissemination was determined by harvesting mice at 1, 7, 21 and 56 days post-vaccination and plating serial dilutions of homogenates of spleen, lungs and liver onto supplemented 7H10 medium. For immunogenicity studies, C57BL/6 mice (fifteen per group) were immunized subcutaneously with 5 × 106 CFU of rBCG::Ag85B-Rv3425 or BCG in 100 μl PBS. Mice were sacrificed to conduct the analysis of immune responses at 3, 6, 9 and 12 weeks after immunization. The experiment was repeated twice.
ELISPOT assays for IFN-γ from spleen cell cultures
3, 6, 9 and 12 weeks after vaccination respectively, mice were killed and their spleens removed aseptically in RPMI-1640 medium containing 10% fetal calf serum (FCS), 2 mM glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin. Spleens were gently grinded through a 70-μm cell strainer, and then single-cell suspensions were prepared with Lympholyte-M density gradient centrifugation (Cedar lane lab, Burlington, NC, USA). Cells were counted and plated at 5 × 105 cells per well in the same medium as already described. The mouse interferon (IFN)-γ ELISPOT kit (U-Cytech Biosciences, CT317-PR5, the Netherlands) was used to determine the relative number of IFN-γ-expressing cells in the single-cell spleen suspensions following the manufacturer’s instructions. The spots were counted microscopically. Wells with fewer than 10 spots were not used for calculations.
Antigen-specific serum antibody titration by ELISA
Sera were collected from the immunized mice at 3, 6, 9, 12 weeks after immunization and stored at −80°C. ELISA plates (Maxisorb, type 96F; Nunc, Roskilde, Denmark) were coated with either protein Ag85B (0.5 μg/ml well) or Rv3425(0.5 μg/ml well) and incubated overnight at 4°C. The plates were blocked with 350 μl per well PBS containing 1% bovine serum albumin for 2 h at 37°C and washed with PBS containing 0.05% Tween-20 three times. Serially diluted sera were added to the wells (beginning at a 1/500 dilution) for 1 h at 37°C and washed, followed by adding 150 μl per well horseradish peroxidase-conjugated rabbit antimouse immunoglobulin G (IgG; Dingguo Biotechnology, Beijing, China), IgG1 and IgG2a (SouthernBiotech, Birmingham, USA) diluted at 1/10,000, 1/1,000 and 1/1,000 in PBS respectively. Plates were incubated for 1 h at 37°C, washed with PBS containing 0.05% Tween-20 three times, and developed with 0.1 M citrate-phosphate buffer, pH 5.0 containing 1 mg/ml o-phenylenediamine (OPD) and 0.03% hydrogen peroxide. After 30 min at room temperature, reactions were stopped by adding (50 μl per well) 1 N H2SO4 and were measured at 492 nm with an ELISA reader. Antibody titers are expressed as reciprocal end point titers.
Data analysis
The difference comparison was carried out with the Student’s t test. Data are expressed as the mean ± standard deviation (SD), and the difference was considered statistically significant when P values was less than 0.05.
Results
Construction of BCG recombinants that secreted Ag85B and Rv3425
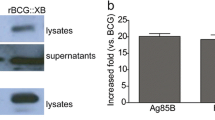
To obtain a new recombinant BCG capable of expressing Ag85B and Rv3425, the two genes were inserted into mycobacterial–E. coli shuttle vector pMV261. The resulting recombinant clones (pMV261-Ag85B-Rv3425 and pMV261) were designated as rBCG::Ag85B-Rv3425 and rBCG::pMV261, respectively. Because of the signal sequence of the Ag85B, the expression of target proteins could be detected in the culture filtrates with two different antibodies. Analysis of the culture filtrates of rBCG::Ag85B-Rv3425 using antibodies recognizing both components revealed a fusion protein of the expected molecular weight, and this was absent from rBCG::pMV261 (Fig. 1). Figure 1a showed that rBCG::Ag85B-Rv3425 expressed and secreted a relatively higher level of Ag85B than BCG. Introduction of the Ag85B and Rv3425 coding regions into BCG resulted in a 50KDa product in culture filtrate of rBCG::Ag85B-Rv3425, which was recognized by the anti-Rv3425 rabbit polyclonal anti-sera (Fig. 1b).
Evaluation the secretion level of IFN-γ in vitro
Single splenocyte suspensions from immunized mice were obtained and assayed for IFN-γ at 72 h post-stimulation. An ELISPOT assay was used to determine the relative numbers of IFN-γ expressing cells in single cell spleen suspensions of mice immunized with rBCG::Ag85B-Rv3425, and BCG. We found that splenocytes from mice vaccinated with rBCG::Ag85B-Rv3425 or BCG produced IFN-γ when stimulated in vitro with protein PPD, Ag85B, Rv3425 or ESAT6 protein(6 μg/ml, which is not over-expressed in the recombinant BCG as the negative re-stimulation control) (Fig. 2). However, the secretion level of IFN-γ in response to PPD, Ag85B or Rv3425 proteins (2 μg/ml), respectively, was increased 3–7 fold in cultures of spleen cells from C57BL/6 mice 6 weeks after rBCG::Ag85B-Rv3425 immunization compared to mice immunized with BCG (Fig. 2).
The celluar immune response was measured in an ELISPOT assay with splenocytes isolated from C57BL/6 mice immunized with rBCG::Ag85B-Rv3425 and BCG. Freshly isolated spleen cells were plated in duplicate at 5 × 105 cell per well in 96 spot and incubated with PPD (2 μg/ml), Ag85B (2 μg/ml), Rv3425 (2 μg/ml) and negative control (ESAT6, 6 μg/ml) for 48 h at 37°C, 5% CO2, respectively. The data are representative of two separate experiments. *P < 0.05 vs. BCG
Evaluation of antigen-specific serum antibody response
Mice were killed for evaluation of antibody responses. Antibody responses were evaluated by ELISA after 3, 6, 9, 12 weeks respectively. Figure 3 illustrates the level of antibody response in the sera of mice from different groups against recombinant purified Ag85B or Rv3425 protein. Compared with the PBS control group, mice vaccinated either with BCG or rBCG::Ag85B-Rv3425 strains induced higher levels of antibody against Ag85B protein. However, IgG antibody titers as high as 1:25,600 were obtained in mice immunized with rBCG::Ag85B-Rv3425 during the 6–9 weeks, which is significantly higher than that induced by immunization with BCG (1:6,400, P < 0.05) (Fig. 3a). The antibody titers of the PBS control group were only 1:50(data not shown in the Fig. 3a). Figure 3c illustrates that the anti-Rv3425 titers of rBCG::Ag85B-Rv3425 were significantly higher than that of BCG (p < 0.05).
Antibody response against Ag85B and Rv3425 in mice immunized with rBCG::Ag85B-Rv3425 or BCG. Sera were collected from mice immunized with rBCG::Ag85B-Rv3425 (triangle), BCG (square) at different weeks (3, 6, 9, 12 weeks) after immunization, then examined for IgG (a, c) and the ratio of IgG2a/IgG1 (b, d). Shown are the means ± standard deviations
Figure 3b and d illustrates the antibody levels of IgG1 and IgG2a isotype against the protein Ag85B and Rv3425, respectively. The ratios of IgG2a/IgG1 were calculated to determine the induction of Th1/Th2 responses in animals. As a result in response to protein Ag85B, the ratio of mice immunized with rBCG::Ag85B-Rv3425 was higher than that of BCG, and at the 9th week the ratio peaked and kept until the end of the experiment. Since the gene RV3425 does not exist in BCG Danish strain, there were no obvious IgG1 and IgG2a titers detected in the mice immunized with BCG Danish strain. So we only showed the ratios of IgG2a/IgG1 from mice immunized with rBCG::Ag85B-Rv3425 in Fig. 3d. The whole results revealed that the capability of the induction of Th1 protection immune response was rBCG::Ag85B-Rv3425 > BCG in the whole experiment.
In vivo persistence of rBCG::Ag85B-Rv3425 is similar to conventional BCG
The immunogenicity of rBCG::Ag85B-Rv3425 could relate to a difference in the persistence of the recombinant strain within the host. To investigate this, we determined if expression of the Ag85B-Rv3425 fusion protein influenced the in vivo growth of BCG. Mice were immunized i.v. with rBCG::Ag85B-Rv3425 and BCG, and the growth in the spleen, lungs and liver was monitored. There was a steady decline in bacterial load over time for two strains, such that numbers were reduced by approximately 2 log in all organs by day 56 post-vaccination (Fig. 4). In all cases, the bacterial load did not differ significantly between rBCG::Ag85B-Rv3425 and BCG, irrespective of the time after vaccination. This suggests that the higher immunogenicity of rBCG::Ag85B-Rv3425 was not due to higher bacterial load, but was related directly to Rv3425 or Ag85B expression in BCG.
In vivo growth of BCG expressing the Ag85B-Rv3425 fusion protein. C57BL/6 mice (n = 4) were immunized i.v. with 5 × 106 CFU of BCG or rBCG::Ag85B-Rv3425. At 1, 7, 21 and 56 days post-vaccination, the bacterial load was assessed in the lung (a), spleen (b) and liver (c). Data are expressed as the mean CFU ± SD from one of two representative experiments
Discussion
BCG, the only vaccine currently available against M. tuberculosis, has highly variable efficacy in protection against adult pulmonary TB. Several explanations for its variable efficacy have been proposed, such as the influence of prior infection on mycobacteria and the absence of antigens that are protective against MTB. Comparative genomics has shown that up to 16 genomic deletions designated RD1–RD16 was found in different BCG sub-strains relative to MTB [21]. These genes are present in virulent mycobacteria but deleted during the attenuation and repeated passages of BCG. Some of these genes are likely associated with virulence and could play an important role for the failure of BCG. Those ORFs absent from M. bovis may be of considerable practical utility. Reintroducing selected genes from RD1 to RD16 to BCG has therefore been suggested as a way towards enhancing the protective efficacy of the existing BCG vaccine. The RD1 region is the best characterized of the deleted regions. This gene segment is found to be absent from all BCG sub-strains and most environmental mycobacteria but is present in all strains within the M. tuberculosis complex [22]. Reintroduction of the RD1 region into BCG led to a protein expression profile almost identical to that of virulent M. bovis and M. tuberculosis as assessed by proteomics [22]. It was reported that mice and guinea pig vaccinated with recombinant strain BCG::RD1-2F9 were better protected against challenge with M. tuberculosis, as compared with control animals immunized with BCG alone [17]. Recombinant BCG expressing and secreting the immunodominant antigen of M. tuberculosis, Ag85B, was recently found to promote levels of protection greater than conventional BCG [16, 23]. These reports demonstrate that modifying BCG by over-expressing important genes (even genes already in BCG) may be a useful approach for improving vaccine performance.
The PE and PPE families, two large unrelated protein families, occupy 10% of the coding capacity of the M. tuberculosis genome. Although their exact functions are unknown, a similar example has been observed where unknown surface antigens of other pathogens that are targets for the host’s immune response have been used as vaccine candidates [24]. As DNA vaccine, Rv3812 and Rv1806–1807 gave a level of protection that was statistically better than saline in the lungs [25]. Three proteins of PE and PPE families (Rv3018c, Rv1818c and Rv3812) of M. tuberculosis have also shown to induce T cell responses in mice [26]. These data indicate that at least some of the genes belonging to this family are expressed by M. tuberculosis in vivo and could induce potential protective immune response against M. tuberculosis.
In this study, we used BCG-Danish as a parental strain to develop a rBCG vector and evaluated for its ability to induce antigen-specific immune responses in mice. In sera of mice immunized with rBCG::Ag85B-Rv3425, the levels of Ag85B-specific and Rv3425-specific IgG were higher than that with BCG. Furthermore, Ag85B-specific IgG titers decreased after 12 weeks, while Rv3425-specific IgG titers increased gradually during immunization process. The results suggested that recombinant BCG over-expressing Ag85B and Rv3425 could prolong antigen-specific immune response compared to BCG. The IgG2a isotype is associated with a Th1-type cytokine response during which IFN-γ is produced. In contrast to BCG, the ratios (IgG2a/IgG1) of mice immunized with rBCG::Ag85B-Rv3425 were higher whatever against the protein Ag85B or Rv3425, which indicate a shift towards a Th1 immune response. Our results showed that rBCG::Ag85B-Rv3425 favored a Th1 response by inducing both higher IFN-γ and IgG2a titers. This is consistent with previous report showing that IFN-γ is involved in differential isotype secretion, since it enhances IgG2a while suppressing IgG1 production [27].
The development of a Th1 immune response mediated by IFN-γ is a prerequisite for mounting efficient protection against M. tuberculosis challenge [28]. Cytokine secretion pattern from rBCG::Ag85B-Rv3425 immunized mice showed higher level of IFN-γ, further confirming the strong Th1 type of response induced by rBCG::Ag85B-Rv3425. Recombinant BCG will retain the attributes of BCG as a vaccine, including a long standing safety profile, a single inoculum, superb adjuvant activity, inexpensive, easily produced and conveniently stored. Hence it can not easily be replaced by other vaccine candidate although the controversies surrounding its use. Improvement of BCG remains amongst the best choices for the rational design of a vaccine in the case of tuberculosis. Our results showed that the better immunogenicity of rBCG::Ag85B-Rv3425 than that of BCG and their similar growth pattern in the organs of mice may make rBCG::Ag85B-Rv3425 the preferred vaccine candidate against TB.
Any new vaccine against tuberculosis would ideally yield improved protective efficacy and not display increased virulence as compared to BCG. Therefore, in the next stage, the protective efficacy of the rBCG::Ag85B-Rv3425 against tuberculosis, the influence of dose and route of immunization will be evaluated with the animal model in ABSL-3 laboratory of the Animal Facility at Wuhan University.
References
Agger EM, Andersen P (2002) A novel TB vaccine; towards a strategy based on our understanding of BCG failure. Vaccine 21:7–14
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell BG (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. doi:10.1038/31159 Medline
Ohara N, Yamada T (2001) Recombinant BCG vaccines. Vaccine 19:4089–4098. doi:10.1016/S0264–410X(01)00155-4 Medline
Hovav AH, Mullerad J, Davidovitch L, Fishman Y, Bigi F, Cataldi A, Bercovier H (2003) The Mycobacterium tuberculosis recombinant 27-Kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protectio. Infect Immun 71:3146–3154. doi:10.1128/IAI.71.6.3146-3154.2003 Medline
Neeraj D, Vivek R, Anil KT (2003) Skewing of the Th1/Th2 responses in mice due to variation in the level of expression of an antigen in a recombinant BCG system. Immunol Lett 88:175–184. doi:10.1016/S0165-2478(03)00043-9 Medline
Horwitz MA (1997) A new TB vaccine. Immunologist 5:15–20
Hess J, Kaufmann SHE (1993) Vaccination strategies against intracellular microbes. FEMS Microbiol Immunol 7:95–103. doi:10.1111/j.1574-695X.1993.tb00387.x
Wiker HG, Harboe M (1992) The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev 56:648–661 Medline
Dhar N, Rao V, Tyagi AK (2004) Immunogenicity of recombinant BCG vaccine strains overexpressing components of the antigen 85 complex of Mycobacterium tuberculosis. Med Microbiol Immunol 193:19–25. doi:10.1007/s00430-002-0170-x
Baldwin SL, D’Souza C, Roberts AD, Kelly BP, Frank AA, Lui MA, Ulmer JB, Huygen K, McMurray DM, Orme IM (1998) Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun 66:2951–2959 Medline
Horwitz MA, Lee BWE, Dillon BJ, Harth G (1995) Protective immunity against tuberculosis induced by vaccination with major extracellular proteins against Mycobacterium tuberculosis. Proc Natl Acad Sci USA 92:1530–1534. doi:10.1073/pnas.92.5.1530 Medline
Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM (1999) Comparative genomics of BCG vaccines by whole genome DNA microarray. Science 284:1520–1523. doi:10.1126/science.284.5419.1520 Medline
Choudhary RK, Mukhopadhyay S, Chakhaiyar P, Sharma M, Murthy KLR, Katoch VM, Hasnain SE (2003) PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect Immun 71:6338–6343. doi:10.1128/IAI.71.11.6338-6343.2003 Medline
Zhang H, Wang J, Lei J, Zhang M, Yang Y, Chen Y, Wang H (2007) PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a potential B-cell antigen used for serological diagnosis to distinguish vaccinated controls from tuberculosis patients. Clin Microbiol Infect 13(2):139–145. doi:10.1111/j.1469-0691.2006.01561.x Medline
Norazmi MN, Mustaffa M (2004) Approaches towards the development of a vaccine against tuberculosis: recombinant BCG and DNA vaccine. Tuberculosis 84:102–109. doi:10.1016/j.tube.2003.08.011 Medline
Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic’ S (2000) Recombinant bacillus Calmette–Gu’erin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci USA 97:13853–13858. doi:10.1073/pnas.250480397 Medline
Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Grifftths KE, Marchal G, Leclerc C, Cole ST (2003) Recombinant BCG exportiong ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9:533–539. doi:10.1038/nm859 Medline
Palendira U, Spratt JM, Britton WJ, Triccas JA (2005) Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol Mycobacterium tuberculosis infection. Vaccine 23:1680–1685. doi:10.1016/j.vaccine.2004.10.007 Medline
Stover CK, Bansal GP, Hanson MS, Burlein JE, Palaszynski SR, Young JF, Koenig S, Young DB, Sadziene A, Barbour AG (1993) Protective immunity elicited by recombinant Bacille Calmette-Guerin(BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J Exp Med 178:197–209. doi:10.1084/jem.178.1.197 Medline
Bao L, Chen W, Zhang HD, Wang XY (2003) Virulence, immunogenicity, and protective efficacy of two recombinant Mycobacterium bovis Bacillus Calmette-Guérin-tupe strains expressing the antigen ESAT-6 from Mycobacterium tuberculosis. Infect Immun 71:1656–1661. doi:10.1128/IAI.71.4.1656-1661.2003 Medline
Brosch R, Gordon SV, Pym A, Eiglmeier K, Garnier T, Cole ST (2000) Comparative genomics of the mycobacteria. Int J Med Microbiol 290:143–152 Medline
Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK (1996) Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol 178:1274–1282 Medline
Xu Y, Zhu BD, Wang QZ, Chen JZ, Qie YQ, Wang JL, Wang HY, Wang BL, Wang HH (2007) Recombinant BCG coexpressing Ag85B, ESAT-6 and mouse-IFN-γ confers effective protection against Mycobacterium tuberculosis in C57BL/6mice. FEMS Immunol Med Microbiol 51:480–487. doi:10.1111/j.1574-695X.2007.00322.x Medline
Howard RJ (1987) Vaccination against malaria: recent advances and the problems of antigenic diversity and other parasite evasion mechanisms. Int J Parasitol 17(1):17–29. doi:10.1016/0020-7519(87)90023-3 Medline
Vipond J, Vipond R, Clark Allen-VercoeE, SO HatchGJ, Gooch KE, Bacon J, Hampshire T, Shuttleworth H, Minton NP, Blake K, Williams A, Marsh PD (2006) Selection of novel TB vaccine candidates and their evaluation as DNA vaccines against aerosol challenge. Vaccine 24:6340–6350. doi:10.1016/j.vaccine.2006.05.025 Medline
Chaitra MG, Nayak R, Shaila MS (2007) Modulation of immune responses in mice to recombinant antigens from PE and PPE families of proteins of Mycobacterium tuberculosis by the Ribi adjuvant. Vaccine 25:7168–7176. doi:10.1016/j.vaccine.2007.07.026 Medline
Jurado A, Carballido J, Griffel H, Hochkeppel HK, Wetzel GD (1989) The immunomodulatory effects of interferon-gamma on mature B lymphocyte responses. Experientia 45:521–526. doi:10.1007/BF01990501 Medline
Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ (1998) Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun 66:830–834 Medline
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program) (2006AA02Z445 and 2006AA02Z420).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiu ling Wang and Ya qing Qie are contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wang, J.l., Qie, Y.q., Zhu, B.d. et al. Evaluation of a recombinant BCG expressing antigen Ag85B and PPE protein Rv3425 from DNA segment RD11 of Mycobacterium tuberculosis in C57BL/6 mice. Med Microbiol Immunol 198, 5–11 (2009). https://doi.org/10.1007/s00430-008-0098-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-008-0098-x