Abstract
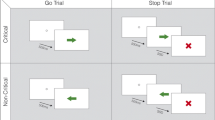
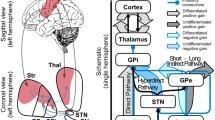
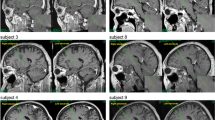
Deep brain stimulation of the dorsal pallidum (globus pallidus, GP) is increasingly considered as a surgical therapeutic option in Huntington’s disease (HD), but there is need to identify outcome measures useful for clinical trials. Computational models consider the GP to be part of a basal ganglia network involved in cognitive processes related to the control of actions. We examined behavioural and event-related potential (ERP) correlates of action control (i.e., error monitoring) and evaluated the effects of deep brain stimulation (DBS). We did this using a standard flanker paradigm and evaluated error-related ERPs. Patients were recruited from a prospective pilot trial for pallidal DBS in HD (trial number NCT00902889). From the initial four patients with Huntington’s chorea, two patients with chronic external dorsal pallidum stimulation were available for follow-up and able to perform the task. The results suggest that the external GP constitutes an important basal ganglia element not only for error processing and behavioural adaptation but for general response monitoring processes as well. Response monitoring functions were fully controllable by switching pallidal DBS stimulation on and off. When stimulation was switched off, no neurophysiological and behavioural signs of error and general performance monitoring, as reflected by the error-related negativity and post-error slowing in reaction times were evident. The modulation of response monitoring processes by GP-DBS reflects a side effect of efforts to alleviate motor symptoms in HD. From a clinical neurological perspective, the results suggest that DBS in the external GP segment can be regarded as a potentially beneficial treatment with respect to cognitive functions.


Similar content being viewed by others
References
Beste C, Saft C, Andrich J, Gold R, Falkenstein M (2006) Error processing in Huntington’s disease. PloS One 1:e86
Beste C, Saft C, Yordanova J, Andrich J, Gold R, Falkenstein M, Kolev V (2007) Functional compensation of pathology in cortico-subcortical interactions in preclinical Huntington’s disease. Neuropsychologia 45:2922–2930
Beste C, Saft C, Konrad C, Andrich J, Habbel A, Schepers I, Jansen A, Pfleiderer B, Falkenstein M (2008) Levels of error processing in Huntington’s disease: a combined study using event-related potentials and voxel-based morphometry. Hum Brain Mapp 29:121–130
Beste C, Domschke K, Kolev V, Yordanova J, Baffa A, Falkenstein M, Konrad C (2010) Functional 5-HT1a receptor polymorphism selectively modulates error-specific subprocesses of performance monitoring. Hum Brain Mapp 31:621–630
Biolsi B, Cif L, Fertit H, Robles SG, Coubes P (2008) Long-term follow-up of Huntington disease treated by bilateral deep brain stimulation of the internal globus pallidus. J Neurosurg 109:130–132
Coles MG, Scheffers MK, Holroyd CB (2001) Why is there an ERN/Ne on correct trials? response representations, stimulus-related components, and the theory of error-processing. Biol Psychol 56:173–189
Crawford JR, Garthwaite PH (2012) Singe-case research in neuropsychology: a comparison of five forms of t-test for comparing a case to controls. Cortex 48:1009–1016
Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK (2005) Trial-by- trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25:11730–11737
Demeestere J, Vandenberghe W (2011) Experimental surgical therapies for Huntington’s disease. CNS Neurosci Ther 17:705–713
Falkenstein M, Hohnsbein J, Hoormann J, Blanke L (1991) Effects of crossmodal divided attention on late ERP components. II. error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78:447–455
Fasano A, Mazzone P, Piano C, Quaranta D, Soleti F, Bentivolglio AR (2008) GPi-DBS in Huntington’s disease: results on motor function and cognition in a 72-year-old case. Mov Disord 23:1289–1292
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward SR, Miller V, Pieper S, Kikinis R (2012) 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 309:1323–1341
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E (1993) A neural system for error detection and compensation. Psychol Sci 4:385–390
Groiss SJ, Elben S, Reck C, Voges J, Wojtecki L, Schnitzler A (2011) Local field potential oscillations of the globus pallidus in Huntington’s disease. Mov Disord 26:2577–2578
Hemm S, Coste J, Gabrillargues J, Ouchchane L, Sarry L, Caire F, Vassal F, Nuti C, Derost P, Durif F, Lemaire J–J (2009) Contact position analysis of deep brain stimulation electrodes on post-operative CT images. Acta Neurochir (Wien) 151:823–829
Hoffmann S, Falkenstein M (2010) Independent component analysis of erroneous and correct responses suggests online response control. Hum Brain Mapp 31:1305–1315
Holroyd CB, Coles MG (2002) The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109:679–709
Humphries MD, Stewart RD, Gurney KN (2006) A physiologically plausible model of action selection and oscillatory activity in the basal ganglia. J Neurosci 26:12921–12942
Huys D, Bartsch C, Poppe P, Lenartz D, Huff W, Prütting J, Timmermann L, Klosterkötter J, Maarouf M, Rommel T, Hartmann A, Sturm V, Kuhn J (2013) Management and outcome of pallidal deep brain stimulation in severe Huntington’s disease. Fortschr Neurol Psychiatry 81:202–205
Jürgens CK, van de Wiel L, van Es AC, Grimbergen YM, Witjes-Ane MN, van der Grond HA, Roos RA (2008) Basal ganglia volume and clinical correlates in preclinical Huntington’s disease. J Neurol 255:1785–1791
Kang GA, Heath S, Rothlind J, Starr PA (2011) Long-term follow-up of pallidal deep brain stimulation in two cases of Huntington’s disease. J Neurol Neurosurg Psychiatry 82:272–277
Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC (2007) Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl 97:561–567
Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, Dagher A, Hutchison WD, Lozano AM (2004) Bilateral globus pallidus stimulation for Huntington’s disease. Ann Neurol 56:290–294
Nguyen L, Bradshaw JL, Stout JC, Croft RJ, Georgiou-Karistianis N (2010) Electrophysiological measures as potential biomarkers in Huntington’s disease: review and future directions. Brain Res Rev 64:177–194
Nunez PL, Pilgreen KL (1991) The spline-laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J Clin Neurophysiol 8:397–413
Politis M, Pavese N, Tai YF, Kiferle L, Mason SL, Brooks DJ, Tabrizi SJ, Barker RA, Piccini P (2011) Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: a multimodal imaging study. Hum Brain Mapp 32:258–270
Siegert S, Herrojo Ruiz M, Brücke C, Huebl J, Schneider G-H, Ullsperger M, Kühn AA (2014) Error signals in the subthalamic nucleus are related to post-error slowing in patients with Parkinson’s disease. Cortex. doi:10.1016/j.cortex.2013.12.008
Smith MA, Brandt J, Shadmehr R (2000) Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature 403:544–549
Spielberger S, Hotter A, Wolf E, Eisner W, Müller J, Poewe W, Seppi K (2012) Deep brain stimulation in Huntington’s disease: a 4-year follow-up case report. Mov Disord 27:806–807
Temel Y, Cao C, Vlamings R, Blokland A, Ozen H, Steinbusch HWM, Michelsen KA, von Hörsten S, Schmitz C, Visser-Vandewalle V (2006) Motor and cognitive improvement by deep brain stimulation in a transgenic rat model of Huntington’s disease. Neurosci Lett 406:138–141
Vidal F, Hasbroucq T, Grapperon J, Bonnet M (2000) Is the error negativity specific to errors? Biol Psychol 51:109–128
Yordanova J, Falkenstein M, Hohnsbein J, Kolev V (2004) Parallel systems of error processing in the brain. Neuroimage 22:590–602
Acknowledgments
This work was supported by a Grant from the Deutsche Forschungsgemeinschaft (DFG) BE4045/10-1. The initial clinical trial was supported with a seed funding by the European Huntington´s Disease Network (EHDN). We thank the patients for their cooperation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Beste, C., Mückschel, M., Elben, S. et al. Behavioral and neurophysiological evidence for the enhancement of cognitive control under dorsal pallidal deep brain stimulation in Huntington’s disease. Brain Struct Funct 220, 2441–2448 (2015). https://doi.org/10.1007/s00429-014-0805-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0805-x