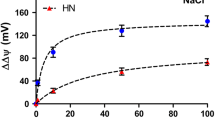
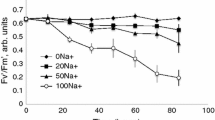
Abstract
The effect of glutamate on plant plasma membrane cation transport was studied in roots of Arabidopsis thaliana (L.) Heynh. Patch-clamp experiments using root protoplasts, 22Na+ unidirectional fluxes into intact roots and measurements of cytosolic Ca2+ activity using plants expressing cytosolically-targeted aequorin in specific cell types were carried out. It was demonstrated that low-millimolar concentrations of glutamate activate within seconds both Na+ and Ca2+ currents in patch-clamped protoplasts derived from roots. The probability of observing glutamate-activated currents increased with increasing glutamate concentration (up to 29% at 3 mM); half-maximal activation was seen at 0.2–0.5 mM glutamate. Glutamate-activated currents were voltage-insensitive, ‘instantaneous’ (completely activated within 2–3 ms of a change in voltage) and non-selective for monovalent cations (Na+, Cs+ and K+). They also allowed the permeation of Ca2+. Half-maximal Na+ currents occurred at 20–30 mM Na+. Glutamate-activated currents were sensitive to non-specific blockers of cation channels (quinine, La3+, Gd3+). Although low-millimolar concentrations of glutamate did not usually stimulate unidirectional influx of 22Na+ into intact roots, they reliably caused an increase in cytosolic Ca2+ activity in protoplasts isolated from the roots of aequorin-transformed Arabidopsis plants. The response of cytosolic Ca2+ activity revealed a two-phase development, with a rapid large transient increase (lasting minutes) and a prolonged subsequent stage (lasting hours). Use of plants expressing aequorin in specific cell types within the root suggested that the cell types most sensitive to glutamate were in the mature epidermis and cortex. The functional significance of these glutamate-activated currents for both cation uptake into plants and cell signaling remains the subject of speculation, requiring more knowledge about the dynamics of apoplastic glutamate in plants.




Similar content being viewed by others
Abbreviations
- GLR :
-
Gene in plants encoding glutamate receptor-like protein
- iGluRs :
-
Ionotropic glutamate receptors
References
Chiu J, DeSalle R, Lam H-M, Maisel L, Coruzzi G (1999) Molecular evolution of glutamate receptors: a primitive signaling mechanism that existed before plants and animals diverged. Mol Biol Evol 16:826–838
Davenport R (2002) Glutamate receptors in plants. Ann Bot 90:549–557
Davenport RJ, Tester M (2000) A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol 122:823–834
Demidchik V, Tester M (2002) Sodium fluxes through non-selective cation channels in the plasma membrane of protoplasts from Arabidopsis thaliana roots. Plant Physiol 128:379–387
Demidchik V, Bowen H, Maathuis F, Shabala S, Tester M, White PJ, Davies J (2002a) Arabidopsis thaliana root nonselective cation channels mediate calcium uptake and are involved in growth. Plant J. 32:799–808
Demidchik V, Davenport RJ, Tester M (2002b) Nonselective cation channels in plants. Annu Rev Plant Biol 53:67–107
Dennison KL, Spalding EP (2000) Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol 124:1511–1514
Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61
Essah PA, Davenport R, Tester M (2003) Sodium influx and accumulation in Arabidopsis thaliana. Plant Physiol 133:307–318
Findlay GP, Tyerman SD, Garrill A, Skerrett M (1994) Pump and K+ inward rectifiers in the plasmalemma of wheat root protoplasts. J Membr Biol 139:103–116
Huettner JE (1990) Glutamate receptor channels in rat DRG neurons—activation by kainate and quisqualate and blockade of desensitization by con-A. Neuron 5:255–266
Kiedrowski L (1999) N-methyl-d-aspartate excitotoxicity: relationships among plasma membrane potential, Na+/Ca2+ exchange, mitochondrial Ca2+ overload, and cytoplasmic concentrations of Ca2+, H+ and K+. Mol Pharmacol 56:619–632
Kiegle E, Moore C, Haseloff J, Tester M, Knight M (2000) Cell-type specific calcium responses to drought, NaCl, and cold in Arabidopsis root: a role for endodermis and pericycle in stress signal transduction. Plant J 23:267–278
Kim SA, Kwak JM, Jae SK, Wang MH, Nam HG (2001) Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol 42:74–84
Kinraide TB, Etherton B (1980) Electrical evidence for different mechanisms of uptake for basic, neutral and acidic amino acids in oat coleoptiles. Plant Physiol 65:1085–1089
Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8:489–503
Lacombe B, Becker D, Hedrich R, Chiu J, DeSalle R, Heinemann S, Hollmann M, Kwak J, Le Novere N, Nam HG, Sakmann B, Schroeder JI, Spalding EP, Tester M, Turano FJ, Coruzzi G (2001) On the identity of plant glutamate receptors. Science 292:1486–1487
Lam H-M, Chiu J, Hsieh M-H, Meisel L, Oliviera IC, Shin M, Coruzzi G (1998) Glutamate receptor genes in plants. Nature 396:125–126
Leinderszufall T, Rand MN, Waxman SG, Kocsis JD (1994) Differential role of 2 Ca2+-permeable non-NMDA glutamate channels in rat retinal ganglion cells—kainate-induced cytoplasmic and nuclear Ca2+ signals. J Neurophysiol 72:2503–2516
Lerma J, Paternain AV, Salvador N, Somohano F, Morales M, Casado M (1998) Excitatory amino acid-activated channels. In: Soria B, Cena V (eds) Ion channel pharmacology. Oxford University Press, UK, pp 399–421
Lohaus G, Heldt H-W (1997) Assimilation of gaseous ammonia and the transport of its products in barley and spinach leaves. J Exp Bot 48:1779–1786
Lohaus G, Winter H, Riens B, Heldt H-W (1995) Further studies of the phloem loading process in leaves of barley and spinach—the comparison of metabolite concentrations in the apoplastic compartment with those in the cytosolic compartment and in the sieve tubes. Bot Acta 108:270–275
Lohaus G, Pennewiss K, Sattelmacher B, Hussmann M, Muehling KH (2001) Is the infiltration-centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiol Plant 111:457–465
Lutts S, Majerus V, Kinet JM (1999) NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol Plant 105:450–458
Maathuis FJM, Sanders D (2001) Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol 127:1617–1625
Meldrum BS (2000) Glutamate as a neurotransmitter in the brain. Review of physiology and pathology. J Nutr [Suppl]130:1007–1015
Parker DR, Norvell WA, Chaney RL (1995) GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In: Loeppert RH, Schwab AB, Goldberg S (eds) Chemical equilibrium and reaction models, special publication 42. Soil Science Society of America, Madison, WI, pp 253–269
Pavlovkin J, Ullrich-Eberius CO, Novacký A (1984) Energization of the uptake of neutral, acidic and basic amino acids in root hairs of Trianea bogotensis Karst. Biologia Bratislava 39:683–691
Roberts SK, Tester M (1997) A patch clamp study of Na+ transport in maize roots. J Exp Bot 48:431–440
Ruan Y-L, Patrick JW, Brady CJ (1996) The composition of apoplast fluid recovered from intact developing tomato fruit. Aust J Plant Physiol 23:9-13
Santa-Cruz A, Acosta M, Rus A, Bolarin MC (1999) Short-term salt tolerance mechanisms in differentially salt tolerant tomato species. Plant Physiol Biochem 37:65–71
Tester M (1988) Pharmacology of K+ channels in the plasmalemma of the green alga, Chara corallina. J. Membr Biol 103:159–169
Ward JM, Schroeder JI (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cells. Plant Cell 6:669–683
Acknowledgements
We particularly thank Dr. Julia Davies in our Department for her support of this work in many ways. We also thank other members of our laboratory, Drs. Romola Davenport and Chris Cheffings for helpful advice and comments on the manuscript, Dr. Fouad Lemtiri-Chlieh, Dr. Matt Gilliham and Mr. John Banfield for technical advice, and Dr. Marc Knight (University of Oxford, UK) for supplying the seeds of Arabidopsis expressing aequorin constitutively. Financial support from a Royal Society/NATO Fellowship to V.D.; Churchill College Studentship and an Overseas Research Students award to P.A.E.; BBSRC Research Development Fellowship to M.T. is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demidchik, V., Essah, P.A. & Tester, M. Glutamate activates cation currents in the plasma membrane of Arabidopsis root cells. Planta 219, 167–175 (2004). https://doi.org/10.1007/s00425-004-1207-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1207-8