Abstract
Introduction
Intramedullary nails for fixation of extracapsular hip fractures have gained popularity recently. Although clinically successful, they are not devoid of complications. An infrequently reported complication is the medial migration of the femoral neck element (FNE) of the implant into the pelvis. The purpose of this study was to create a biomechanical model simulating this effect based on a clinical case radiographic analysis.
Methods
Eight clinical cases of medial migration were available for radiographic analysis. Medial migration was quantified and the fractures were classified. A biomechanical model was built comprising two fixtures containing the nail and FNE respectively. A pivot between the two fixtures, representing a deficient femoral calcar, simulated an unstable fracture type. Two pivot points were used for each nail. The constructs were tested using sinusoidal loading (40–800 N at 2 Hz) and medial migration was assessed. Five different nail designs (TFN, PFN, PFN-a, Gamma-3 and IMHS) were tested (overall 75 tests).
Results
All the five implants demonstrated medial migration to a similar distance. The TFN required the highest number of cycles (3127 ± 2569) and the IMHS the lowest (58.8 ± 3.6) although this difference did not reach statistical significance (P = 0.07). Changing the pivot point for the medial calcar did not alter the results significantly. All eight clinical cases demonstrated an unstable intertrochanteric fracture pattern (AO/OTA 32A2).
Conclusions
Discrete biomechanical conditions are required to reproduce medial migration of the FNE in cephalomedullary devices.
Similar content being viewed by others
Introduction
Intramedullary nails for fixation of pertrochanteric fractures have gained popularity recently, and have several potential advantages over sliding hip screws, particularly in unstable fractures [1, 3, 9, 14, 20]. The biomechanical advantages include a shorter lever arm and reducing the bending moment on the implant, while still allowing controlled impaction of the fracture [9, 11]. Various implant types are available, some with significantly different designs. One of the fundamental features in these different implant designs is the type of fixation in the femoral head, which includes single or dual-lag screws, screws sliding through a barrel, or new “blade-type” designs [5, 10]. The most common and well-documented mechanical complication of these devices is cut-out of the hip screw through the femoral head, with varus collapse of the fracture, which was previously modeled in the laboratory [17]. Another complication described in early designs is the fracture of the femoral shaft distal to the tip of the implant. Newer nail designs have addressed this issue and reduced the risk for this latter phenomenon [9, 14]. A third and rarely reported mechanism of implant failure is the medial migration of the femoral neck element into the pelvis. This has been termed the “Z-effect” and has been described primarily for two-screw devices such as the proximal femoral nail (PFN, Synthes, Switzerland) [21], but has occurred in implants with a single femoral head fixation element as well, including the trochanteric fixation nail (TFN), Gamma, Zimmer, and Depuy nails [2, 4, 6, 7, 8, 13, 18, 19]. The incidence of this phenomenon remains unknown and the biomechanical explanation for the medial migration of the femoral neck element has not been elucidated in the literature. The purpose of this study was to present a biomechanical model that recreates medial migration in the laboratory setting, and provide a testing apparatus for different nail systems, with an attempt to offer a possible explanation for the clinical phenomenon. In addition, a few available clinical cases were analyzed radiographically to shed some more light on this rare phenomenon.
Materials and methods
Biomechanical hypothesis
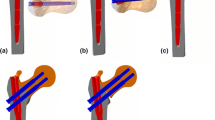
Based on the radiographic findings (Table 1) in a series of eight cases of pertrochanteric fractures in which medial migration occurred following utilization of the TFN (Synthes, Paoli, PA, USA), a consistent pattern was observed. The greater trochanter was not intact in seven out of the eight cases and the medial cortical buttress was compromised. Our hypothesis was that the lack of a proximal buttress for the proximal part of the nail as well as an unstable calcar support, often seen in these fractures, enabled a toggling or rocking motion of the proximal part of the nail in the coronal plane. During weight bearing, the femoral neck element is loaded medially along its axis while the nail toggles. If this load overcomes the resistance of the bone to implant interface at the medial part of the implant, the femoral neck element may migrate slightly medially. As the load on the femur is reduced, the nail can drop back laterally, but friction between the femoral head and the femoral neck element may hold the latter in place while the nail resets to its original position. As this cycle is repeated and this ratcheting effect propagates, the femoral neck element may be continually driven further into the femoral head (Fig. 1a, b).
We also believe that an important contributor to the phenomenon is varying bone density throughout different locations in the femoral head. First the bone must be more resistant to cut-out superiorly than medially, else the FNE would more likely cut out superiorly. Also, since the FNE cuts through medially each cycle, but then is held in its new position, there must be a source of friction elsewhere in the femoral head or neck holding the FNE in place, overcoming the friction between the FNE and the nail, or else the FNE would toggle back with the nail. If this friction were too high, it would not allow the blade to cut through, and if it were too low, it would not hold the FNE in the new position. A biomechanical test model was developed that encompassed these theories to replicate the mechanism in different implants.
Biomechanical model
In order to test the above hypothesis a mechanical model recreating medial migration of the femoral neck element was designed.
Implants
Five intramedullary nail devices designed for extracapsular hip fracture fixation from different manufacturers and designs were used for the study: the TFN (Synthes, Paoli, PA, USA) with a blade device for the femoral head, 11 mm in diameter, 170 mm long, and a 130° neck-shaft angle; the PFN with two hip screws (Synthes, Switzerland), 11 mm in diameter, 240 mm long, and a 130° angle; the PFN-a representing a non-round femoral neck implant (Synthes, Switzerland), 10 mm in diameter, 240 mm long, and a 130° angle; the Gamma-3 nail (Stryker Howmedica, NJ), 10.5 mm in diameter, 180 mm long, and a 130° angle; and the IMHS (Smith and Nephew, Memphis, TN, USA) nail with a barrel through which the FNE slides, 12 mm in diameter nail, 210 mm long, and a 130° angle (Fig. 2). All lag screws and blades were modified by cutting off the threaded or helical blade portion and replacing it with a smooth shaft in the same diameter. This was done in order to allow the same controlled friction to be applied to all implants, preventing the threads or blade flutes from cutting through the plastic part of the simulator and eliminating additional unknown variables. This is explained and described in detail in the following simulator design section. All neck implants were modified to be 115 mm in length to fit the model.
Simulator design
A metal fixture to accommodate the nail was created (Fig. 3). This component represented the femoral canal. Each nail was fixed distally with a bolt through its distal locking hole according to the manufacturer’s recommendations (5 mm locking bolt for each design except the IMHS which was locked with two 4.5 mm bolts). The lateral side of the fixture had a large hole to accommodate the femoral neck element and simulate deficient lateral cortical support. The medial side of the fixture had a plate with pins allowing the femoral head implant to pivot, upon axial loading simulating an unstable pertrochanteric fracture with lack of medial support. Toggling was enabled by loading of the pivot point axially. In the case of an intact medial support, the inferomedial calcar would have resisted this toggling effect.
The test was run twice with pins in two locations. The axis of the pivot pin was 25 mm medial to the axis of the nail and located either 32 or 37 mm from the top of the nail fixture. All the nail constructs were tested at both pivot pin locations, except for the PFN, which was tested only on the higher pivot pin location due to fixturing limitations of the proximal femoral neck element construct. In between the pivot plate and the nail, a piece of elastomer (3/8″ thick, 70 A durometer) was held in place. The elastomer was used to simulate the elastic properties of the nail/bone interface that allow the nail to return toward its original position as the load is reduced.
The femoral head fixture was composed of a delrin head with a hole specific to the diameter of the neck implant, allowing it to slide back and forth freely, ensuring that there was less resistance to medial migration than superior cutout and controlled simulated bone density. A metal shell encapsulated the plastic head. A delrin bushing was pressed onto the neck implant through a hole in the delrin head via a set screw tightened through the metal shell to a torque of 0.5 Nm using a calibrated torque wrench, creating a frictional hold on the neck implant in the femoral head. The threaded section of the screws and the helical section of the blade were removed and replaced with a smooth shaft. This allowed us to control the frictional hold on the neck implant in the femoral head in a known manner, eliminating unknown, uncontrolled frictional interactions in the simulated head. As described earlier, all implants, whether screws or blades were reported to migrate medially, so it does not seem that the shape or design of current implants inherently prevent migration. Therefore it was best to normalize the test by controlling the friction in the head. This allowed consistency between the various devices (Fig. 4). Clinically this frictional hold on the implant is highly dependent on bone quality and may vary by region in femoral head and neck.
The femoral nail was connected to an arm using the notches at the top of the nail intended for the targeting arm. A linear variable displacement transducer (LVDT) was connected with a cable to the neck implant. The LVDT measured the amount of medial migration in the direction of the axis of the neck element. The entire construct was placed on a tilt table angled at 19° to simulate a loading vector of 149° consistent with the physiological load vector [17]. The final construct (Fig. 5) was axially loaded with an MTS machine, applying a sinusoidal load from 40 to 800 N at 2 Hz. The end point of each testing was medial migration of the neck implant by 9 mm or completion of 5,000 cycles.
Each nail construct was tested three times. The first run was with the pivot plate in the higher of the two locations described, and the second run was with the pivot plate in the lower position. The third time, the elastomer between the pivot plate and the nail was replaced with a set screw, which was tightened flush with the nail and did not allow nail motion. For this third test, the pivot plate was placed in the location that showed the quickest movement of the neck implant. This was to test the null hypothesis, expecting that if the nail did not toggle, no migration was expected. Each of these tests was repeated for five constructs of each implant type. Five TFN constructs, five PFN constructs, five PFN-A constructs, five Gamma-3 constructs, and five IMHS constructs were tested for a total of 75 tests.
Clinical radiographic analysis
Eight hip fracture patients from four centers who had medial migration post TFN fixation were available for analysis. Immediate postoperative and late postoperative films demonstrating the medial migration (range 4–10 weeks) were available for analysis. These fractures were classified using the AO/OTA classification system and a measurement of medial migration was performed by a fellowship trained orthopedic trauma surgeon. Determining the amount of medial migration was done by measuring the change in the distance of the lateral superior aspect of the femoral neck element from the lateral femoral cortex, by calibrating the distance with the known diameter of the superior part of the nail (which is 17 mm), using Adobe Photoshop Version 5.0 software.
Statistics
The ANOVA tests were used to determine differences in means between the study groups. A post hoc Bonferonni test was used to determine differences between the groups. A P value of <0.05 was considered statistically significant. SPPS PC v. 11 (Chicago, IL, USA) was used for statistical processing.
Results
Biomechanical analysis
Medial migration of the femoral neck implant occurred in all five implants. Table 2 specifies the number of cycles required to reach the end-point of each implant while using the pivot point at 32 mm.
The TFN did not complete migration in two out of the five test runs migrating only 6.6 and 3.5 mm and did not migrate at all after applying 5,000 load cycles in one run. Thus the average cycles required for migration for this implant was significantly higher than the other implants (Table 2). There was no significant difference in the average migration distance of all implants.
Changing the pivot point from 32 to 37 mm from the top of the nail did not alter the overall effect of medial migration in the four implants tested (Table 3). Although the TFN exhibited the highest number of cycles and the IMHS exhibited the lowest number of cycles required for medial migration, the differences did not reach statistical significance (P = 0.07, Table 3).
Replacing the elastomer of the fixture with a set screw, preventing nail toggle, completely prevented the medial migration of the femoral neck element of the TFN, PFN-a, Gamma and IMHS. One gamma nail fixture broke before the completion of 5,000 cycles (at 2,983 cycles).
When the PFN was clamped using a set screw to prevent toggling, medial migration of the inferior neck element still occurred in all samples at an average of 551 ± 179 cycles. Therefore, another set of measurements was done with the set screw while removing the smaller, superior neck screw. In that configuration, no migration occurred in any of the five tests after 5,000 cycles.
Radiographic analysis
Seven out of eight fractures were AO/OTA 31-A2 types and one was 31-A3. The average time to failure was 6.5 ± 2.5 weeks (range 4–10). The mean medial migration was 9.6 ± 6.3 mm (range 5.5–22.6, Table 1).
Discussion
Our results demonstrated that using an appropriate biomechanical model, medial migration of the femoral neck element of intramedullary nails for the fixation of pertrochanteric fractures can be reliably reproduced. All implant designs tested demonstrated medial migration of their femoral neck element using this model. This phenomenon, in our opinion, is an inherent property of these devices, regardless of the number of femoral neck elements, different sliding interfaces, or the use of either lag screws or helical blades. The TFN performed slightly better with regard to the medial migration for the first pivot point for unknown reasons, requiring further investigation.
Our model also may imply that certain fracture patterns lead to nail toggling within the femoral canal, including a deficient lateral buttress and an unstable calcar pattern. This was supported in the current study because it was shown that toggling is necessary for medial migration and when toggling was prevented, medial migration did not occur with single FNE. The exception to this rule was the PFN, which has two femoral neck elements. In this case, preventing nail toggle did not prevent medial migration of the distal femoral neck element when two neck implants were used. Migration was prevented only when the nail was clamped and the superior neck element removed. This behavior suggests a different mechanism of migration in two-screw devices that requires further investigation to understand.
In the clinical reports, a similar phenomenon was described as the “Z-effect”, first by Werner [21] but also in subsequent reports [4, 13]. However, when originally reported, the “Z effect” referred to the medial migration of the superior neck screw into the femoral head. The explanation given was that varus loading upon an unstable calcar causes collapse of the fracture and sliding of the inferior neck screw, while the superior neck screw, for unknown reasons, remained in place and protruded into the femoral head. The “reverse-Z” effect, described by Boldin [2] refers to excess lateral sliding of the superior neck element. The differences between the clinical findings and our model might suggest that two-element implants may behave differently due to interaction between the two screws and the nail. This may play a role in creating a different migration pattern. A clue to that might be the fact that upon removal of the superior neck element, the PFN did not differ from the rest of the implants tested. Further biomechanical evaluation is therefore required to delineate these observations.
It appears that the shape of the neck implant or the presence of a sleeve (IMHS) does not prevent the distal migration of the neck implant, and the latter tended to occur in a lower number of cycles. A possible cause may have been lower friction between the nail and neck element created by the sleeve, but further investigation is required to prove this.
Based on the clinical radiographic analysis of eight cases, there are some observations concordant with the biomechanical model described in this study. The most salient radiographic feature of our cases and other reported in the literature, including the “Z effect” reports, involved an unstable fracture of the A2 type, i.e., fracture pattern with medial compromise [2, 8, 9, 13, 18]. Greater trochanteric fractures were also observed, but their role remains uncertain. We could not find a common denominator among the clinical regarding screw length, screw position in orthogonal radiographic views or other surgical technical details. We also could not reach any conclusion regarding the correlation between the anti-rotational mechanism of the implant and the occurrence of migration that was consistently reported in FNE’s using various anti-rotational mechanisms such as set screws (Gamma), antirotational screws (PFN) or a helical blade (TFN) [6, 15, 16, 18]. Also, other inherent properties of the nail such as diameter, number of locking bolts or the interface between the femoral neck-element and the nail (that were not changed during the testing) did not prevent medial migration. Medial migration of the femoral neck element is a rare but troubling phenomenon described in a variety of intramedullary hip fracture implants. Its incidence remains unknown but might be underestimated. In two large recent meta-analyses comparing intramedullary versus extramedullary devices for the treatment of extracapsular hip fractures encompassing around 3,000 patients in each [9, 14], this complication is not mentioned at all. However, when examining some of the studies included in these reports, medial migration of the hip screw does occur and it is mentioned usually as a single event [16, 15] mainly associated with the PFN, but also with the gamma nail [7]. A search of the FDA web site [6] for adverse effects yielded 20 complaints reporting either protrusion or migration of the femoral neck element during the period 1999–2005. These included the Gamma nail, Gamma-3, DePuy Ace, Zimmer ITST and the TFN nails. However, it is inconclusive, based on the description of each report, whether these represent true migration of the screw or simply lack of sufficient implant sliding. Given the fact that over 350,000 hip fractures are treated in the United States alone each year, out of which roughly 50% are extracapsular [12], coupled with the increasing use of intramedullary devices, the low reported incidence might reflect indeed a low incidence. On the other hand, this phenomenon is severe enough to cause significant complications such as penetration of the acetabulum [7, 18] as well as possible bowel and bladder injury [18]. It should be also noted that device manufacturers include mechanisms to prevent complete separation of the femoral neck element from the intramedullary nail. Despite that, medial migration had been described with these implants that were also tested in our study.
Although consistently reproducible, there were some limitations of the model used in this study. First, all threaded elements of the neck implants were removed in order to control the friction in the femoral head. Using our model, a constant friction was applied in the femoral head by using a torque limited set screw. In the clinical setting, however, the actual friction within the femoral head may vary upon the bone quality and the threads of the implant, and may be difficult to predict. Therefore a cadaveric model simulating this effect, although desired, is almost impossible to obtain. Further study is required to determine the friction level required for the phenomenon to occur, and to correlate it with radiographic findings such as the bone density.
In our model, it was impossible to predict the amount of toggling or the exact force required to cause migration. In the clinical situation, many more cycles are probably required to produce the phenomenon since the motion may be minimal. However, our exaggerated rate of toggling had demonstrated a plausible mechanism explaining the main component of medial migration of the FNE in cephalomedullary devices. Further research is also needed to delineate the exact mechanism of medial migration where two neck elements are used such as in the PFN, including the interaction between the two screws and the nail.
Another limitation in our study was the standardization of the FNE done by removal of the threaded element. However, this was required to explore the effect of friction in the femoral head simulating the bone/implant interface in a controlled fashion. Future studies exploring the effect of different implant/bone interface will be needed to assess the influence of the threads or blades on the migration potential.
The development of a model for the medial migration of the neck implant in intramedullary nails used for pertrochanteric fracture fixation will enable manufacturers to evaluate constructs designed to prevent this phenomenon. One would postulate that a system that permits only one-way sliding of the femoral neck element would eliminate this effect.
In our model, many conditions had to be satisfied in order to achieve medial migration. These included lateral buttress deficiency (lateral opening in the fixture), unstable medial cortex (simulated by pivot pins), constant controlled friction within the femoral head and axial loading in varus. Due to the rarity of the phenomenon it is unlikely to pin-point specific fracture and implant positioning patterns predicting medial migration of the femoral neck element. However, there might be a somewhat increased risk for the latter to occur in a case of an unstable fracture pattern with or without a greater trochanteric fracture. In any case, vigilant radiographic follow-up is required for the detection of medial migration of the femoral neck element in patients treated with intramedullary nails for extracapsular hip fractures.
References
Adams CI, Robinson CM, Court-Brown CM, McQueen MM (2001) Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma 15(6):394–400
Boldin C, Seibert FJ, Fankhauser F, Peicha G, Grechenig W, Szyszkowitz R (2003) The proximal femoral nail (PFN)—a minimal invasive treatment of unstable proximal femoral fractures: a prospective study of 55 patients with a follow-up of 15 months. Acta Orthop Scand 74(1):53–58
Crawford CH, Malkani AL, Cordray S, Roberts CS, Sligar W (2006) The trochanteric nail versus the sliding hip screw for intertrochanteric hip fractures: a review of 93 cases. J Trauma 60(2):325–328; discussion 328–329
Fogagnolo F, Kfuri M Jr, Paccola CA (2004) Intramedullary fixation of pertrochanteric hip fractures with the short AO-ASIF proximal femoral nail. Arch Orthop Trauma Surg 124(1):31–37
Gardner MJ, Bhandari M, Lawrence BD, Helfet DL, Lorich DG (2005) Treatment of intertrochanteric hip fractures with the AO trochanteric fixation nail. Orthopedics 28(2):117–122
Health TFaDAcfDaR. Medical device reporting. Available at: http://www.fda.gov/cdrh/mdr/index.html
Hesse B, Gachter A (2004) Complications following the treatment of trochanteric fractures with the gamma nail. Arch Orthop Trauma Surg 124(10):692–698
Hohendorff B, Meyer P, Menezes D, Meier L, Elke R (2005) [Treatment results and complications after PFN osteosynthesis]. Unfallchirurg 108(11):938, 940, 941–936 passim
Jones HW, Johnston P, Parker M (2006) Are short femoral nails superior to the sliding hip screw? A meta-analysis of 24 studies involving 3,279 fractures. Int Orthop 30(2):69–78
Lenich A, Mayr E, Ruter A, Mockl C, Fuchtmeier B (2006) First results with the Trochanter Fixation Nail (TFN): a report on 120 cases. Arch Orthop Trauma Surg 126(10):706–712
Mahomed N, Harrington I, Kellam J, Maistrelli G, Hearn T, Vroemen J (1994) Biomechanical analysis of the Gamma nail and sliding hip screw. Clin Orthop Relat Res (304):280–288
Morris AH, Zuckerman JD (2002) National consensus conference on improving the continuum of care for patients with hip fracture. J Bone Joint Surg Am 84-A(4):670–674
Papasimos S, Koutsojannis CM, Panagopoulos A, Megas P, Lambiris E (2005) A randomised comparison of AMBI, TGN and PFN for treatment of unstable trochanteric fractures. Arch Orthop Trauma Surg 125(7):462–468
Parker MJ, Handoll HH (2005) Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev (Online) (4):CD000093
Saudan M, Lubbeke A, Sadowski C, Riand N, Stern R, Hoffmeyer P (2002) Pertrochanteric fractures: is there an advantage to an intramedullary nail?: a randomized, prospective study of 206 patients comparing the dynamic hip screw and proximal femoral nail. J Orthop Trauma 16(6):386–393
Schipper IB, Steyerberg EW, Castelein RM, van der Heijden FH, den Hoed PT, Kerver AJ, van Vugt AB (2004) Treatment of unstable trochanteric fractures. Randomised comparison of the gamma nail and the proximal femoral nail. J Bone Joint Surg 86(1):86–94
Sommers MB, Roth C, Hall H, Kam BC, Ehmke LW, Krieg JC, Madey SM, Bottlang M (2004) A laboratory model to evaluate cutout resistance of implants for pertrochanteric fracture fixation. J Orthop Trauma 18(6):361–368
Tauber M, Resch H (2006) Sigmoid perforation after medial migration of lag screw in gamma nailing. Arch Orthop Trauma Surg 126(2):118–122
Tyllianakis M, Panagopoulos A, Papadopoulos A, Papasimos S, Mousafiris K (2004) Treatment of extracapsular hip fractures with the proximal femoral nail (PFN): long term results in 45 patients. Acta Orthop Belg 70(5):444–454
Utrilla AL, Reig JS, Munoz FM, Tufanisco CB (2005) Trochanteric gamma nail and compression hip screw for trochanteric fractures: a randomized, prospective, comparative study in 210 elderly patients with a new design of the gamma nail. J Orthop Trauma 19(4):229–233
Werner-Tutschku W, Lajtai G, Schmiedhuber G, Lang T, Pirkl C, Orthner E (2002) [Intra- and perioperative complications in the stabilization of per- and subtrochanteric femoral fractures by means of PFN]. Unfallchirurg 105(10):881–885
Acknowledgements
Conflict of interests: Drs Helfet, Gardner and Weil had not received any compensation or support for their participation in the study. Mr Pierson and Mikhail are employees of Synthes Research Facility, West Chester, PA, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weil, Y.A., Gardner, M.J., Mikhail, G. et al. Medial migration of intramedullary hip fixation devices: a biomechanical analysis. Arch Orthop Trauma Surg 128, 227–234 (2008). https://doi.org/10.1007/s00402-007-0497-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-007-0497-2