Abstract
Purpose
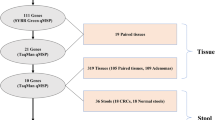
The aims of this study were to investigate the use of quantitative CGI methylation data from stool DNA to classify colon cancer patients and to relate stool CGI methylation levels to those found in corresponding tissue samples.
Methods
We applied a quantitative methylation-specific PCR assay to determine CGI methylation levels of six genes, previously shown to be aberrantly methylated during colorectal carcinogenesis. Assays were performed on DNA from biopsies of “normal” mucosa and stool samples from 57 patients classified as disease-free, adenoma, or cancer by endoscopy, and in tumour tissue from cancer patients. Additionally, CGI methylation was analysed in stool DNA from an asymptomatic population of individuals covering a broad age range (mean = 47 ± 24 years)
Results
CGI methylation levels in stool DNA were significantly higher than in DNA from macroscopically normal mucosa, and a significant correlation between stool and mucosa was observed for ESR1 only. Multivariate statistical analyses using the methylation levels of each CGI in stool DNA as a continuous variable revealed a highly significant (p = 0.003) classification of cancer vs. non-cancer (adenoma + disease-free) patients (sensitivity = 65 %, specificity = 81 %).
Conclusion
CGI methylation profiling of stool DNA successfully identified patients with cancer despite the methylation status of CGIs in stool DNA not generally reflecting those in DNA from the colonic mucosa.



Similar content being viewed by others
References
Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH, Rosen L, Zapka JG, Olsen SJ, Giardiello FM, Sisk JE, Van Antwerp R, Brown-Davis C, Marciniak DA, Mayer RJ (1997) Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 112(2):594–642. doi:10.1053/gast.1997.v112.agast970594
Ahlquist DA, Wieand HS, Moertel CG, McGill DB, Loprinzi CL, O'Connell MJ, Mailliard JA, Gerstner JB, Pandya K, Ellefson RD (1993) Accuracy of fecal occult blood screening for colorectal neoplasia. A prospective study using Hemoccult and HemoQuant tests. JAMA 269(10):1262–1267. doi:10.1001/jama.269.10.1262
Allison JE, Feldman R, Tekawa IS (1990) Hemoccult screening in detecting colorectal neoplasm: sensitivity, specificity, and predictive value. Long-term follow-up in a large group practice setting. Ann Intern Med 112(5):328–333
Allison JE, Tekawa IS, Ransom LJ, Adrain AL (1996) A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 334(3):155–159. doi:10.1056/NEJM199601183340304
Lieberman DA, Weiss DG (2001) One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 345(8):555–560. doi:10.1056/NEJMoa010328
Young GP, John DJBS, Winawer SJ, Rozen P (2002) Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies. a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol 97(10):2499–2507. doi:10.1111/j.1572-0241.2002.06046.x
Sidransky D, Tokino T, Hamilton SR, Kinzler KW, Levin B, Frost P, Vogelstein B (1992) Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science 256(5053):102–105. doi:10.1126/science.1566048
Villa E, Dugani A, Rebecchi A, Vignoli A, Grottola A, Buttafoco P, Losi L, Perini M, Trande P, Merighi A, Lerose R, Manenti F (1996) Identification of subjects at risk for colorectal carcinoma through a test based on K-ras determination in the stool. Gastroenterology 110(5):1346–1353. doi:10.1053/gast.1996.v110.pm8613038
Traverso G, Shuber A, Levin B, Johnson C, Olsson L, Schoetz DJ Jr, Hamilton SR, Boynton K, Kinzler KW, Vogelstein B (2002) Detection of APC mutations in fecal DNA from patients with colorectal tumors. N Engl J Med 346(5):311–320. doi:10.1056/NEJMoa012294
Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME, the Colorectal Cancer Study G (2004) Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 351(26):2704–2714. doi:10.1056/NEJMoa033403
Ahlquist DA, Sargent DJ, Loprinzi CL, Levin TR, Rex DK, Ahnen DJ, Knigge K, Lance MP, Burgart LJ, Hamilton SR, Allison JE, Lawson MJ, Devens ME, Harrington JJ, Hillman SL (2008) Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med 149(7):441–450, W481
Belinsky SA, Nikula KJ, Palmisano WA, Michels R, Saccomanno G, Gabrielson E, Baylin SB, Herman JG (1998) Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci U S A 95(20):11891–11896. doi:10.1073/pnas.95.20.11891
Enokida H, Shiina H, Urakami S, Igawa M, Ogishima T, Li L-C, Kawahara M, Nakagawa M, Kane CJ, Carroll PR, Dahiya R (2005) Multigene methylation analysis for detection and staging of prostate cancer. Clin Cancer Res 11(18):6582–6588. doi:10.1158/1078-0432.ccr-05-0658
Fackler MJ, McVeigh M, Mehrotra J, Blum MA, Lange J, Lapides A, Garrett E, Argani P, Sukumar S (2004) Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res 64(13):4442–4452. doi:10.1158/0008-5472.can-03-3341
Grote HJ, Schmiemann V, Geddert H, Bocking R, Kappes R, Gabbert HE, Sarbia M (2006) Methylation of RAS association domain family protein 1A as a biomarker of lung cancer. Canc Cytopathol 108(2):129–134. doi:10.1002/cncr.21717
Jubb AM, Quirke P, Oates AJ (2003) DNA methylation, a biomarker for colorectal cancer: implications for screening and pathological utility. Ann NY Acad Sci 983(1):251–267
Kawamoto K, Enokida H, Gotanda T, Kubo H, Nishiyama K, Kawahara M, Nakagawa M (2006) p16INK4a and p14ARF methylation as a potential biomarker for human bladder cancer. Biochem Biophys Res Commun 339(3):790–796. doi:10.1016/j.bbrc.2005.11.072
Belshaw NJ, Elliott GO, Williams EA, Bradburn DM, Mills SJ, Mathers JC, Johnson IT (2004) Use of DNA from human stools to detect aberrant CpG island methylation of genes implicated in colorectal cancer. Canc Epidemiol Biomarkers Prev 13(9):1495–1501
Chang E, Il Park D, Kim YJ, Kim BK, Park JH, Kim HJ, Cho YK, Il Sohn C, Jeon WK, Kim BI, Kim HD, Kim DH, Kim YH (2010) Detection of colorectal neoplasm using promoter methylation of ITGA4, SFRP2, and p16 in stool samples: a preliminary report in Korean patients. Hepato-Gastroenterol 57(101):720–727
Chen WD, Han ZJ, Skoletsky J, Olson J, Sah J, Myeroff L, Platzer P, Lu S, Dawson D, Willis J, Pretlow TP, Lutterbaugh J, Kasturi L, Willson JK, Rao JS, Shuber A, Markowitz SD (2005) Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Canc Inst 97(15):1124–1132. doi:10.1093/jnci%2Fdji204
Glockner SC, Dhir M, Yi JM, McGarvey KE, Van Neste L, Louwagie J, Chan TA, Kleeberger W, de Bruine AP, Smits KM, Khalid-de Bakker CAJ, Jonkers DMAE, Stockbrugger RW, Meijer GA, Oort FA, Iacobuzio-Donahue C, Bierau K, Herman JG, Baylin SB, Van Engeland M, Schuebel KE, Ahuja N (2009) Methylation of TFPI2 in stool DNA: a potential novel biomarker for the detection of colorectal cancer. Cancer Res 69(11):4691–4699. doi:10.1158/0008-5472.can-08-0142
Huang ZH, Li LH, Wang JF (2007) Hypermethylation of SFRP2 as a potential marker for stool-based detection of colorectal cancer and precancerous lesions. Dig Dis Sci 52(9):2287–2291. doi:10.1007/s10620-007-9755-y
Huang ZH, Li LH, Yang F, Wang JF (2007) Detection of aberrant methylation in fecal DNA as a molecular screening tool for colorectal cancer and precancerous lesions. World J Gastroenterol 13(6):950–954
Lenhard K, Bommer GT, Asutay S, Schauer R, Brabletz T, Goke B, Lamerz R, Kolligs FT (2005) Analysis of promoter methylation in stool: a novel method for the detection of colorectal cancer. Clin Gastroenterol Hepatol 3(2):142–149. doi:10.1016/S1542-3565(04)00624-X
Leung WK, To KF, Man EP, Chan MW, Bai AH, Hui AJ, Chan FK, Lee JF, Sung JJ (2004) Detection of epigenetic changes in fecal DNA as a molecular screening test for colorectal cancer: a feasibility study. Clin Chem 50(11):2179–2182. doi:10.1373/clinchem.2004.039305
Leung WK, To K-F, Man EPS, Chan MWY, Hui AJ, Ng SSM, Lau JYW, Sung JJY (2007) Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in fecal samples of patients with colorectal cancer or polyps. Am J Gastroenterol 102(5):1070–1076. doi:10.1111/j.1572-0241.2007.01108.x
Muller HM, Oberwalder M, Fiegl H, Morandell M, Goebel G, Zitt M, Muhlthaler M, Ofner D, Margreiter R, Widschwendter M (2004) Methylation changes in faecal DNA: a marker for colorectal cancer screening? Lancet 363(9417):1283–1285
Oberwalder M, Zitt M, Wontner C, Fiegl H, Goebel G, Kohle O, Muhlmann G, Ofner D, Margreiter R, Muller HM (2008) SFRP2 methylation in fecal DNA: a marker for colorectal polyps. Int J Color Dis 23:15–19. doi:10.1007/s00384-007-0355-2
Petko Z, Ghiassi M, Shuber A, Gorham J, Smalley W, Washington MK, Schultenover S, Gautam S, Markowitz SD, Grady WM (2005) Aberrantly methylated CDKN2A, MGMT, and MLH1 in colon polyps and in fecal DNA from Patients with colorectal polyps. Clin Cancer Res 11(3):1203–1209
Wang DR, Tang D (2008) Hypermethylated SFRP2 gene in fecal DNA is a high potential biomarker for colorectal cancer noninvasive screening. World J Gastroenterol 14(4):524–531. doi:10.3748/wjg.14.524
Ahlquist D, Skoletsky J, Boynton K, Harrington J, Mahoney D, Pierceall W, Thibodeau S, Shuber A (2000) Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology 119(5):1219–1227. doi:10.1053/gast.2000.19580
Klaassen CHW, Jeunink MAF, Prinsen CFM, Ruers TJM, Tan ACITL, Strobbe LJA, Thunnissen FBJM (2003) Quantification of human DNA in feces as a diagnostic test for the presence of colorectal cancer. Clin Chem 49(7):1185–1187. doi:10.1373/49.7.1185
Belshaw NJ, Elliott GO, Foxall RJ, Dainty JR, Pal N, Coupe A, Garg D, Bradburn DM, Mathers JC, Johnson IT (2008) Profiling CpG island field methylation in both morphologically normal and neoplastic human colonic mucosa. Br J Cancer 99(1):136–142. doi:10.1038/sj.bjc.6604432
Ahuja N, Issa JP (2000) Aging, methylation and cancer. Histol Histopathol 15(3):835–842
Ahuja N, Li Q, Mohan AL, Baylin SB, Issa J-PJ (1998) Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res 58(23):5489–5494
Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA (2001) Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res 61(9):3573–3577
Worthley DL, Whitehall VLJ, Buttenshaw RL, Irahara N, Greco SA, Ramsnes I, Mallitt KA, Le Leu RK, Winter J, Hu Y, Ogino S, Young GP, Leggett BA (2010) DNA methylation within the normal colorectal mucosa is associated with pathway-specific predisposition to cancer. Oncogene 29(11):1653–1662. doi:10.1038/onc.2009.449
Nakagawa H, Nuovo GJ, Zervos EE, Martin EW, Salovaara R, Aaltonen LA, de la Chapelle A (2001) Age-related hypermethylation of the 5′ region of MLH1 in normal colonic mucosa is associated with microsatellite-unstable colorectal cancer development. Cancer Res 61(19):6991–6995
Kang MY, Lee BB, Ji YI, Jung EH, Chun H-K, Song SY, Park S-E, Park J, Kim D-H (2008) Association of interindividual differences in p14ARF promoter methylation with single nucleotide polymorphism in primary colorectal cancer. Cancer 112(8):1699–1707. doi:10.1002/cncr.23335
Lind GE, Thorstensen L, Lovig T, Meling GI, Hamelin R, Rognum TO, Esteller M, Lothe RA (2004) A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol Canc 3:28
Konishi K, Shen L, Jelinek J, Watanabe Y, Ahmed S, Kaneko K, Kogo M, Takano T, Imawari M, Hamilton SR, Issa J-PJ (2009) Concordant DNA methylation in synchronous colorectal carcinomas. Canc Prev Res 2(9):814–822. doi:10.1158/1940-6207.capr-09-0054
Moriyama T, Matsumoto T, Nakamura S, Jo Y, Mibu R, Yao T, Iida M (2007) Hypermethylation of p14(ARF) may be predictive of colitic cancer in patients with ulcerative colitis. Dis Colon Rectum 50(9):1384–1392
Saito S, Kato J, Hiraoka S, Horii J, Suzuki H, Higashi R, Kaji E, Kondo Y, Yamamoto K (2011) DNA methylation of colon mucosa in ulcerative colitis patients: correlation with inflammatory status. Inflamm Bowel Dis:n/a-n/a. doi:10.1002/ibd.21573
Team RDC (2006) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Feinberg AP, Ohlsson R, Henikoff S (2006) The epigenetic progenitor origin of human cancer. Nat Rev Genet 7(1):21–33. doi:10.1038/nrg1748
Ahlquist DA, Klatt KK, Harrington JJ, Cunningham JM (2002) Novel use of hypermethylated DNA markers in stool for detection of colorectal cancer: a feasibility study. Gastroenterology 122(suppl 1):A40
Boynton KA, Summerhayes IC, Ahlquist DA, Shuber AP (2003) DNA Integrity as a potential marker for stool-based detection of colorectal cancer. Clin Chem 49(7):1058–1065. doi:10.1373/49.7.1058
Zou H, Harrington JJ, Klatt KK, Ahlquist DA (2006) A sensitive method to quantify human long DNA in stool: relevance to colorectal cancer screening. Canc Epidemiol Biomarkers Prev 15(6):1115–1119. doi:10.1158/1055-9965.epi-05-0992
Hellebrekers DMEI, Lentjes MHFM, van den Bosch SM, Melotte V, Wouters KAD, Daenen KLJ, Smits KM, Akiyama Y, Yuasa Y, Sanduleanu S, Khalid-de Bakker CAJ, Jonkers D, Weijenberg MP, Louwagie J, van Criekinge W, Carvalho B, Meijer GA, Baylin SB, Herman JG, de Bruïne AP, van Engeland M (2009) GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res 15(12):3990–3997. doi:10.1158/1078-0432.ccr-09-0055
Azuara D, Rodriguez-Moranta F, de Oca J, Soriano-Izquierdo A, Mora J, Guardiola J, Biondo S, Blanco I, Peinado MA, Moreno V, Esteller M, Capella G (2010) Novel methylation panel for the early detection of colorectal tumors in stool DNA. Clin Colorectal Canc 9(3):168–176. doi:10.3816/CCC.2010.n.023
Loktionov A (2007) Cell exfoliation in the human colon: myth, reality and implications for colorectal cancer screening. Int J Cancer 120(11):2281–2289. doi:10.1002/ijc.22647
Rosen K, Shi W, Calabretta B, Filmus J (2002) Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand. A novel mechanism of Anoikis of intestinal epithelial cells. J Biol Chem 277(48):46123–46130. doi:10.1074/jbc.M207883200
Fenton RG, Hixon JA, Wright PW, Brooks AD, Sayers TJ (1998) Inhibition of Fas (CD95) expression and Fas-mediated apoptosis by oncogenic Ras. Cancer Res 58(15):3391–3400
Peli J, Schroter M, Rudaz C, Hahne M, Meyer C, Reichmann E, Tschopp J (1999) Oncogenic Ras inhibits Fas ligand-mediated apoptosis by downregulating the expression of Fas. EMBO J 18(7):1824–1831. doi:10.1093/emboj/18.7.1824
Acknowledgments
We thank the UK Food Standards Agency (project no. N12009) and the Biotechnology and Biological Sciences Research Council (42212A) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elliott, G.O., Johnson, I.T., Scarll, J. et al. Quantitative profiling of CpG island methylation in human stool for colorectal cancer detection. Int J Colorectal Dis 28, 35–42 (2013). https://doi.org/10.1007/s00384-012-1532-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1532-5