Abstract
Purpose
We report the effect of antiangiogenic therapy on the biodistribution of 18F-FPPRGD2 (a surrogate biomarker of integrin αvβ3 expression), and the potential of 18F-FPPRGD2 to predict the prognosis in patients with cervical cancer and ovarian cancer in this clinical scenario.
Methods
Data from six women, age range 30 – 59 years (mean ± SD 44.0 ± 12.5 years), who had undergone a 18F-FPPRGD2 PET/CT scan and bevacizumab-containing therapy were prospectively collected and analyzed. We compared baseline 18F-FPPRGD2 and 18F-FDG uptake in the lesions and tumor-to-background (T/B) ratios. The maximum and mean 18F-FPPRGD2 standardized uptake values (SUVmax and SUVmean) were recorded for 13 normal organs, as well as in all the identified malignant lesions on the pretreatment scan and the 1-week post-treatment scan. We also measured changes in 18F-FPPRGD2 uptake from before to 1 week after treatment, and compared them to the changes in 18F-FDG uptake from before to 6 weeks after treatment. Treatment outcomes were correlated with these changes.
Results
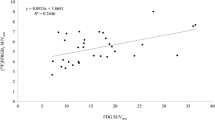
The uptake in lesions and T/B ratio of 18F-FPPRGD2 were lower than those of 18F-FDG (SUVmax 3.7 ± 1.3 vs. 6.0 ± 1.8, P < 0.001; SUVmean 2.6 ± 0.7 vs. 4.2 ± 1.3, P < 0.001; T/B ratio based on SUVmax 2.4 ± 1.0 vs. 2.6 ± 1.0, P < 0.04; T/B ratio based on SUVmean 1.9 ± 0.6 vs. 2.4 ± 1.0, P < 0.003). One patient did not return for the follow-up scan and in another patient no lesions were identified on the pretreatment scan. 18F-FPPRGD2 uptake in lesions in the remaining four patients had significantly changed 1 week after treatment (SUVmean 3.3 ± 1.0 vs. 2.7 ± 1.0, P < 0.001), while uptake in all normal tissues analyzed was not affected by treatment. One patient with clinical disease progression had a decrease in lesional 18F-FPPRGD2 SUVmean of 1.6 % and in 18F-FDG SUVmean of 9.4 %. Two patients with a clinical complete response to treatment had decreases in lesional 18F-FPPRGD2 SUVmean of 25.2 % and 25.0 % and in 18F-FDG SUVmean of 6.1 % and 71.8 %. One patient with a clinical partial response had a decrease in lesional 18F-FPPRGD2 SUVmean of 7.9 % and in 18F-FDG SUVmean of 76.4 %.
Conclusion
This pilot study showed that 18F-FPPRGD2 and 18F-FDG provide independent information about the biology of ovarian and cervical cancers. Bevacizumab-containing therapy does not affect 18F-FPPRGD2 uptake in normal organs, but does result in statistically significant changes in lesions. In addition, 18F-FPPRGD2 may have potential for early prediction of response to such treatments. These preliminary findings have to be confirmed in larger studies.




Similar content being viewed by others
References
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57.
Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–21.
Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71.
Max R, Gerritsen RR, Nooijen PT, Goodman SL, Sutter A, Keilholz U, et al. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71:320–4.
Wu WB, Peng HC, Huang TF. Disintegrin causes proteolysis of beta-cateninand apoptosis of endothelial cells. Involvement of cell-cell and cell-ECM interactions in regulating cell viability. Exp Cell Res. 2003;286:115–27.
Okada Y, Copeland BR, Hamann GF, Koziol JA, Cheresh DA, del Zoppo GJ. Integrin alphavbeta3 is expressed in selected microvessels after focal cerebral ischemia. Am J Pathol. 1996;149:37–44.
Eliceiri BP, Cheresh DA. Role of alpha v integrins during angiogenesis. Cancer J. 2000;6 Suppl 3:S245–9.
Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–8.
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–64.
Shannon KE, Keene JL, Settle SL, Duffin TD, Nickols MA, Westlin M, et al. Anti-metastatic properties of RGD-peptidomimetic agents S137 and S247. Clin Exp Metastasis. 2004;21:129–38.
Haubner R, Wester HJ. Radiolabeled tracers for imaging of tumor angiogenesis and evaluation of anti-angiogenic therapies. Curr Pharm Des. 2004;10:1439–55.
Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49 Suppl 2:113S–28S.
Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin Cancer Res. 2006;12:3942–9.
Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nährig J, Watzlowik P, et al. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med. 2008;49:255–9.
Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ, et al. Comparison of integrin alphaVbeta3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49:22–9.
Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005;2:e70.
Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–86.
Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc. 2004;126:5730–9.
Liu S, Liu Z, Chen K, Yan Y, Watzlowik P, Wester HJ, et al. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol Imaging Biol. 2010;12:530–8.
Mittra ES, Goris ML, Iagaru AH, Kardan A, Burton L, Berganos R, et al. Pilot pharmacokinetic and dosimetric studies of (18)F-FPPRGD2: a PET radiopharmaceutical agent for imaging α(v)β(3) integrin levels. Radiology. 2011;260:182–91.
Iagaru A, Mosci C, Shen B, Chin FT, Mittra E, Telli ML, et al. 18F-FPPRGD2 PET/CT: pilot phase evaluation of breast cancer patients. Radiology. 2014;273:549–59.
Iagaru A, Mosci C, Mittra E, Zaharchuk G, Fischbein N, Harsh G, et al. Glioblastoma multiforme recurrence: an exploratory study of (18)F FPPRGD2 PET/CT. Radiology. 2015;277:497–506.
Minamimoto R, Jamali M, Barkhodari A, Mosci C, Mittra E, Shen B, et al. Biodistribution of the (18)F-FPPRGD2 PET radiopharmaceutical in cancer patients: an atlas of SUV measurements. Eur J Nucl Med Mol Imaging. 2015;42:1850–8.
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83.
Stockler MR, Hilpert F, Friedlander M, King MT, Wenzel L, Lee CK, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32:1309–16.
Tewari KS, Sill MW, Long 3rd HJ, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–43.
Chin FT, Shen B, Liu S, Berganos RA, Chang E, Mittra E, et al. First experience with clinical-grade ([18F]FPP(RGD2): an automated multi-step radiosynthesis for clinical PET studies. Mol Imaging Biol. 2012;14:88–95.
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82.
Morrison MS, Ricketts SA, Barnett J, Cuthbertson A, Tessier J, Wedge SR. Use of a novel Arg-Gly-Asp radioligand, 18F-AH111585, to determine changes in tumor vascularity after antitumor therapy. J Nucl Med. 2009;50:116–22.
Liu Z, Liu S, Wang F, Liu S, Chen X. Noninvasive imaging of tumor integrin expression using (18)F-labeled RGD dimer peptide with PEG (4) linkers. Eur J Nucl Med Mol Imaging. 2009;36:1296–307.
Giatromanolaki A, Koukourakis MI, Theodossiou D, Barbatis K, O'Byrne K, Harris AL, et al. Comparative evaluation of angiogenesis assessment with anti-factor-VIII and anti-CD31 immunostaining in non-small cell lung cancer. Clin Cancer Res. 1997;3:2485–92.
Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 2009;106:10666–71.
Acknowledgments
We thank our research coordinators, the radiochemistry staff, and the nuclear medicine technologists. We particularly thank all the patients who agreed to participate in the study and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Mary Lake Polan Endowment supported the research activities of O.D. and this project.
Conflict of interest
S.S.G. Activities related to the present article: none to disclose. Activities not related to the present article: on the board of Endra, Enlight, ImaginAB, MagArray, SiteOne Therapeutics, VisualSonics/Sonosite, and Click Diagnostics; consultant for VisualSonics/Sonosite, Gamma Medica, BMEB, and Bracco; received grants from General Electric and Sanofi-Aventis; received honoraria from Imaging AB; holds stock in Enlight and VisualSonics/Sonosite; received compensation for travel and accommodations from Gamma Camera.
A.I. Activities related to the present article: none to disclose. Activities not related to the present article: received grants from GE Healthcare and Bayer Healthcare.
The other authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research support
Department discretionary funds (O.D.).
Rights and permissions
About this article
Cite this article
Minamimoto, R., Karam, A., Jamali, M. et al. Pilot prospective evaluation of 18F-FPPRGD2 PET/CT in patients with cervical and ovarian cancer. Eur J Nucl Med Mol Imaging 43, 1047–1055 (2016). https://doi.org/10.1007/s00259-015-3263-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3263-7