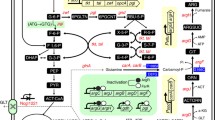
Abstract
Carbohydrates exclusively serve as feedstock for industrial amino acid production with Corynebacterium glutamicum. Due to the industrial interest, knowledge about the pathways for carbohydrate metabolization in C. glutamicum steadily increases, enabling the rational design of optimized strains and production processes. In this review, we provide an overview of the metabolic pathways for utilization of hexoses (glucose, fructose), disaccharides (sucrose, maltose), pentoses (d-ribose, l-arabinose, d-xylose), gluconate, and β-glucosides present in C. glutamicum. Recent approaches of metabolic engineering of l-lysine production strains based on the known pathways are described and evaluated with respect to l-lysine yields.

Similar content being viewed by others
References
Arndt A, Eikmanns BJ (2008) Regulation of carbon metabolism in Corynebacterium glutamicum. In: Burkovski A (ed) Corynebacteria: genomics and molecular biology. Caister Academic, Norfolk, pp 155–182
Bäumchen C, Krings E, Bringer S, Eggeling L, Sahm H (2009) Myo-inositol facilitators IolT1 and IolT2 enhance D-mannitol formation from d-fructose in Corynebacterium glutamicum. FEMS Microbiol Lett 290:227–235
Barrett E, Stanton C, Zelder O, Fitzgerald G, Ross RP (2004) Heterologous expression of lactose- and galactose-utilizing pathways from lactic acid bacteria in Corynebacterium glutamicum for production of lysine in whey. J Bacteriol 70:2861–2866
Becker J, Klopprogge C, Zelder O, Heinzle E, Wittmann C (2005) Amplified expression of fructose 1, 6-bisphosphatase in Corynebacterium glutamicum increases in vivo flux through the pentose phosphate pathway and lysine production on different carbon sources. Appl Environ Microbiol 71:8587–8596
Becker J, Klopproge C, Herold A, Zelder O, Bolten CJ, Wittmann C (2007) Metabolic flux engineering of l-lysine production in Corynebacterium glutamicum—over expression and modification of G6P dehydrogenase. J Biotechnol 132:99–109
Becker J, Klopprogge C, Schröder H, Wittmann C (2009) Metabolic engineering of the tricarboxylic acid cycle for improved lysine production by Corynebacterium glutamicum. Appl Environ Microbiol 75:7866–7869
Blombach B, Schreiner ME, Moch M, Oldiges M, Eikmanns BJ (2007) Effect of pyruvate dehydrogenase complex deficiency on l-lysine production with Corynebacterium glutamicum. Appl Microbiol Biotechnol 76:615–623
Blombach B, Arndt A, Auchter M, Eikmanns BJ (2009) l-Valine production during growth of pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum in the presence of ethanol or by inactivation of the transcriptional regulator SugR. Appl Environ Microbiol 75:1197–1200
Brabetz W, Liebl W, Schleifer KH (1991) Studies on the utilization of lactose by Corynebacterium glutamicum, bearing the lactose operon of Escherichia coli. Arch Microbiol 155:607–612
Brinkrolf K, Plöger S, Solle S, Brune I, Nentwich SS, Hüser AT, Kalinowski J, Pühler A, Tauch A (2008) The LacI/GalR family transcriptional regulator UriR negatively controls uridine utilization of Corynebacterium glutamicum by binding to catabolite-responsive element (cre)-like sequences. Microbiology 154:1068–1081
Cremer J, Eggeling L, Sahm H (1991) Control of the lysine biosynthesis sequence in Corynebacterium glutamicum as analyzed by overexpression of the individual corresponding genes. Appl Environ Microbiol 57:1746–1752
Dominguez H, Lindley ND (1996) Complete sucrose metabolism requires fructose phosphotransferase activity in Corynebacterium glutamicum to ensure phosphorylation of liberated fructose. Appl Environ Microbiol 62:3878–3880
Dominguez H, Rollin C, Guyonvarch A, Guerquin-Kern JL, Cocaign-Bousquet M, Lindley ND (1998) Carbon-flux distribution in the central metabolic pathways of Corynebacterium glutamicum during growth on fructose. Eur J Biochem 254:96–102
Eikmanns BJ (1992) Identification, sequence analysis, and expression of a Corynebacterium glutamicum gene cluster encoding the three glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase, 3-phosphoglycerate kinase, and triosephosphate isomerase. J Bacteriol 174:6076–6086
Engels V, Wendisch VF (2007) The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J Bacteriol 189:2955–2966
Engels V, Georgi T, Wendisch VF (2008) ScrB (Cg2927) is a sucrose-6-phosphate hydrolase essential for sucrose utilization by Corynebacterium glutamicum. FEMS Microbiol Lett 289:80–89
Frunzke J, Engels V, Hasenbein S, Gätgens C, Bott M (2008) Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol Microbiol 67:305–322
Georgi T, Rittmann D, Wendisch VF (2005) Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1, 6-bisphosphatase. Metab Eng 7:291–301
Gourdon P, Raherimandimby M, Dominguez H, Cocaign-Bousquet M, Lindley ND (2003) Osmotic stress, glucose transport capacity and consequences for glutamate overproduction in Corynebacterium glutamicum. J Biotechnol 104:77–85
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104:155–172
Ikeda M, Nakagawa S (2003) The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62:99–109
Jojima T, Omumasaba CA, Inui M, Yukawa H (2010) Sugar transporters in efficient utilization of mixed sugar substrates: current knowledge and outlook. Appl Microbiol Biotechnol 85:471–480
Kabus A, Georgi T, Wendisch VF, Bott M (2007) Expression of the Escherichia coli pntAB genes encoding a membrane-bound transhydrogenase in Corynebacterium glutamicum improves l-lysine formation. Appl Microbiol Biotechnol 75:47–53
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Krämer R, Linke B, McHardy AC, Meyer F, Möckel B, Pfefferle W, Pühler A, Rey DA, Rückert C, Rupp O, Sahm H, Wendisch VF, Wiegräbe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
Kawaguchi H, Vertès AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72:3418–3428
Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H (2007) Engineering of an l-arabinose metabolic pathway in Corynebacterium glutamicum. Appl Microbiol Biotechnol 77:1053–1062
Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H (2009) Identification and functional analysis of the gene cluster for l-arabinose utilization in Corynebacterium glutamicum. Appl Environ Microbiol 75:3419–3429
Kelle R, Hermann T, Bathe B (2005) l-Lysine production. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC, Boca Raton, pp 465–488
Kiefer P, Heinzle E, Zelder O, Wittmann C (2004) Comparative metabolic flux analysis of lysine-producing Corynebacterium glutamicum cultured on glucose or fructose. Appl Environ Microbiol 70:229–239
Kotrba P, Inui M, Yukawa H (2001) The ptsI gene encoding enzyme I of the phosphotransferase system of Corynebacterium glutamicum. Biochem Biophys Res Commun 289:1307–1313
Kotrba O, Inui M, Yukawa H (2003) A single V317A or V317M substitution in enzyme II of a newly identified β-glucoside phosphotransferase and utilization system of Corynebacterium glutamicum R extends its specificity towards cellobiose. Microbiology 149:1569–1580
Krause FS, Henrich A, Blombach B, Krämer R, Eikmanns BJ, Seibold GM (2010) Increased glucose utilization in Corynebacterium glutamicum by use of maltose, and its application for the improvement of l-valine productivity. Appl Environ Microbiol 76:370–374
Lee JK, Sung MH, Yoon KH, Yu JH, Oh TK (1994) Nucleotide sequence of the gene encoding the Corynebacterium glutamicum mannose enzyme II and analyses of the deduced protein sequence. FEMS Microbiol Lett 119:137–145
Lee HW, Pan JG, Lebeault JM (1998) Enhanced l-lysine production in the threonine-limited continuous culture of Corynebacterium glutamicum by using gluconate as a secondary carbon source with glucose. Appl Microbiol Biotechnol 49:9–15
Letek M, Valbuena N, Ramos A, Ordóñez E, Gil JA, Mateos LM (2006) Characterization and use of catabolite-repressed promoters from gluconate genes in Corynebacterium glutamicum. J Bacteriol 188:409–423
Liebl W (2005) Corynebacterium taxonomy. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC, Boca Raton, pp 9–34
Marx A, de Graaf AA, Wiechert W, Eggeling L, Sahm H (1996) Determination of the fluxes in the central metabolism of Corynebacterium glutamicum by nuclear magnetic resonance spectroscopy combined with metabolite balancing. Biotechnol Bioeng 49:111–129
Marx A, Striegel K, de Graaf AA, Eggeling L (1997) Response of central metabolism of Corynebacterium glutamicum to different flux burdens. Biotechnol Bioeng 56:168–180
Marx A, Hans S, Möckel B, Bathe B, de Graaf AA (2003) Metabolic phenotype of phosphoglucose isomerase mutants of Corynebacterium glutamicum. J Biotechnol 104:185–197
Mitsuhashi S, Hayashi M, Ohnishi J, Ikeda M (2006) Disruption of malate:quinone oxidoreductase increases l-lysine production by Corynebacterium glutamicum. Biosci Biotechnol Biochem 70:2803–2806
Moon MW, Kim HJ, Oh TK, Shin CS, Lee JS, Kim SJ, Lee JK (2005) Analyses of enzyme II gene mutants for sugar transport and heterologous expression of fructokinase gene in Corynebacterium glutamicum ATCC 13032. FEMS Microbiol Lett 244:259–266
Moritz B, Striegel K, de Graaf AA, Sahm H (2000) Kinetic properties of the glucose-6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their application for predicting pentose pathway flux in vivo. Eur J Biochem 267:3442–3452
Moritz B, Striegel K, de Graaf AA, Sahm H (2002) Changes of pentose phosphate pathway flux in vivo in Corynebacterium glutamicum during leucine-limited batch cultivation as determined from intracellular metabolite concentration measurements. Metab Eng 4:295–305
Nakayama K, Tanaka H, Hagino H, Kinoshita S (1966) Studies on lysine fermentation. V. Concerted feedback inhibition of aspartokinase and the absence of lysine inhibition on aspartic semialdehyde-pyruvate condensation in Micrococcus glutamicus. Agric Biol Chem 30:611–616
Nentwich SS, Brinkrolf K, Gaigalat L, Hüser AT, Rey DA, Mohrbach T, Marin K, Pühler A, Tauch A, Kalinowski J (2009) Characterization of the LacI-type transcriptional repressor RbsR controlling ribose transport in Corynebacterium glutamicum ATCC 13032. Microbiology 155:150–164
Ohnishi J, Mitsuhashi S, Hayashi M, Ando S, Yokoi H, Ochiai K, Ikeda M (2002) A novel methodology employing Corynebacterium glutamicum genome information to generate a new l-lysine producing mutant. Appl Microbiol Biotechnol 58:217–223
Ohnishi J, Katahira R, Mitsuhashi S, Kakita S, Ikeda M (2005) A novel gnd mutation leading to increased l-lysine production in Corynebacterium glutamicum. FEMS Microbial Lett 242:265–274
Omumasaba CA, Okai N, Inui M, Yokawa H (2004) Corynebacterium glutamicum glyceraldehyde-3-phosphate dehydrogenase isoforms with opposite, ATP-dependent regulation. J Mol Microbiol Biotechnol 8:91–103
Parche S, Burkovski A, Sprenger GA, Weil B, Krämer R, Titgemeyer F (2001) Corynebacterium glutamicum: a dissection of the PTS. J Mol Microbiol Biotechnol 3:423–428
Park SY, Kim HK, Yoo SK, Oh TK, Lee JK (2000) Characterization of glk, a gene coding for glucose kinase of Corynebacterium glutamicum. FEMS Microbiol Lett 188:209–215
Pátek M (2007) Branched chain amino acids. In: Wendisch VF (ed) Amino acid biosynthesis—pathways, regulation and metabolic engineering. Springer, Heidelberg, pp 129–162
Peters-Wendisch PG, Schiel B, Wendisch VF, Katsoulidis E, Möckel B, Sahm H, Eikmanns BJ (2001) Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol 3:295–300
Pons A, Dussap CG, Péquignot C, Gros JB (1996) Metabolic flux distribution in Corynebacterium melassecola ATCC 17965 for various carbon sources. Biotechnol Bioeng 51:177–189
Radmacher E, Eggeling L (2007) The three tricarboxylate synthase activities of Corynebacterium glutamicum and increase of l-lysine synthesis. Appl Microbiol Biotechnol 76:587–595
Riedel C, Rittmann D, Dangel P, Möckel B, Sahm H, Eikmanns BJ (2001) Characterization, expression, and inactivation of the phosphoenolpyruvate carboxykinase gene from Corynebacterium glutamicum and significance of the enzyme for growth and amino acid production. J Mol Microbiol Biotechnol 3:573–583
Rittmann D, Schaffer S, Wendisch VF, Sahm H (2003) Fructose-1, 6-bisphosphatase from Corynebacterium glutamicum: expression and deletion of the fbp gene and biochemical characterization of the enzyme. Arch Microbiol 180:285–292
Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H (2008) Simultaneous utilization of d-cellobiose, d-glucose, and d-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 81:691–699
Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H (2009) Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl Microbiol Biotechnol 85:105–115
Schrumpf B, Eggeling L, Sahm H (1992) Isolation and prominent characteristics of an l-lysine hyperproducing strain of Corynebacterium glutamicum. Appl Microbiol Biotechnol 37:566–571
Seibold G, Auchter M, Berens S, Kalinowski J, Eikmanns BJ (2006) Utilization of soluble starch by a recombinant Corynebacterium glutamicum strain: growth and lysine production. J Biotechnol 124:381–391
Seibold GM, Wurst M, Eikmanns BJ (2009) Roles of maltodextrin and glycogen phosphorylases in maltose utilization and glycogen metabolism in Corynebacterium glutamicum. Microbiology 155:347–358
Shiio I, Miyajima R (1969) Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium flavum. J Biochem (Tokyo) 5:849–859
Shiio I, Ozaki H, Ujigawa-Takeda K (1982) Production of aspartic acid and lysine by citrate synthase mutants of Brevibacterium flavum. Agric Biol Chem 46:101–107
Sprenger GA (2007) Aromatic amino acids. In: Wendisch VF (ed) Amino acid biosynthesis—pathways, regulation and metabolic engineering. Springer, Heidelberg, pp 93–127
Stolz M, Peters-Wendisch P, Etterich H, Gerharz T, Faurie R, Sahm H, Fersterra H, Eggeling L (2007) Reduced folate supply as a key to enhanced l-serine production by Corynebacterium glutamicum. Appl Environ Microbiol 73:750–755
Tanaka Y, Teramoto H, Inui M, Yukawa H (2009) Identification of a second β-glucoside phosphoenolpyruvate:carbohydrate phosphotransferase system in Corynebacterium glutamicum R. Microbiology 155:3652–3660
Tateno T, Fukuda H, Kondo A (2007a) Production of l-lysine from starch by Corynebacterium glutamicum displaying α-amylase on its cell surface. Appl Microbiol Biotechnol 74:1213–1220
Tateno T, Fukuda H, Kondo A (2007b) Direct production of l-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis α-amylase using cspB promoter and signal sequence. Appl Microbiol Biotechnol 77:533–541
Tateno T, Okada Y, Tsuchidate T, Tanaka T, Fukuda H, Kondo A (2009) Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing alpha-amylase and lysine decarboxylase. Appl Microbiol Biotechnol 82:115–121
Udaka S (2008) The discovery of Corynebacterium glutamicum and birth of amino acid fermentation industry in Japan. In: Burkovski A (ed) Corynebacteria: genomics and molecular biology. Caister Academic, Norfolk, pp 1–6
Vallino JJ, Stephanopoulos G (1994) Carbon flux distributions at the glucose-6-phosphate branch point in Corynebacterium glutamicum during lysine overproduction. Biotechnol Prog 10:327–334
Wendisch VF, Bott M, Eikmanns BJ (2006) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr Opin Microbiol 9:268–274
Winnen B, Felce J, Saier MH Jr (2005) Genomic analyses of transport proteins in Corynebacterium glutamicum and Corynebacterium efficiens. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC, Boca Raton, pp 149–186
Yokota A, Lindley ND (2005) Central metabolism: sugar uptake and conversion. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC, Boca Raton, pp 215–240
Yukawa H, Omumasaba CA, Nonaka H, Kós P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertès AA, Inui M (2007) Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058
Acknowledgment
We thank B. J. Eikmanns for valuable discussions and for critically reading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blombach, B., Seibold, G.M. Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of l-lysine production strains. Appl Microbiol Biotechnol 86, 1313–1322 (2010). https://doi.org/10.1007/s00253-010-2537-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2537-z