Abstract
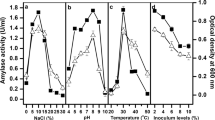
A moderately halophilic alkalitolerant Bacillus sp. Strain TSCVKK, with an ability to produce extracellular halophilic, alkalitolerant, surfactant, and detergent-stable alpha-amylase was isolated from soil samples obtained from a salt-manufacturing industry in Chennai. The culture conditions for higher amylase production were optimized with respect to NaCl, substrate, pH, and temperature. Maximum amylase production of 592 mU/ml was achieved in the medium at 48 h with 10% NaCl, 1% dextrin, 0.4% yeast extract, 0.2% tryptone, and 0.2% CaCl2 at pH 8.0 at 30 °C. The enzyme activity in the culture supernatant was highest with 10% NaCl at pH 7.5 and 55 °C. The amylase that was partially purified by acetone precipitation was highly stable in various surfactants and detergents. Glucose, maltose, and maltooligosaccharides were the main end products of starch hydrolysis indicating that it is an alpha-amylase.




Similar content being viewed by others
References
Amoozegar MA, Malekzadeh F, Malik KA (2003) Production of amylase by newly isolated moderate halophile, Halobacillus sp. Strain MA-2. J Microbiol Methods 52:353–359
Ashger M, Javaid Asad M, Rahman SU, Legge RL (2007) A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. Process Biochem 79:950–955
Bernfeld P (1955) Amylases α and β. Methods Enzymol 1:149–158
Burhan A, Nisa U, Gokhan C, Omer C, Ashabil A, Osman G (2003) Enzymatic properties of novel thermophilic, alkaline and chelator resistant amylases from an alkalophilic Bacillus sp. isolate ANT-6. Process Biochem 38:1397–1403
Coronado M-J, Vargas C, Hofemeister J, Ventosa A, Nieto JJ (2000) Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett 183:67–71
Deutch CE (2002) Characterization of a salt-tolerant extracellular α-amylase from Bacillus dipsosauri. Lett Microbiol 35:78–84
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylase: a biotechnological perspective. Process Biochem 38:1599–1616
Horikoshi K (1999) Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev 63:735–750
Igarashi K, Hatada Y, Hagihara H, Saeki K, Takaiwa M, Uemura T, Ara K, Ozaki K, Kawai S, Kobayashi T, Ito S (1998) Enzymatic properties of a novel liquefying α-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Appl Environ Microbiol 64:3282–3289
Ingvorsen K, Jorgensen BB (1984) Kinetics of sulfate uptake by freshwater and marine species of Desulfovibrio. Arch Microbiol 139:61–66
Joo W-A, Kim C-W (2005) Proteomics of Halophilic archae. J Chromatogr B 815:237–250
Khire JM (1994) Production of moderately halophilic amylase by newly isolated Micrococcus sp. 4 from a salt pan. Lett Appl Microbiol 19:210–212
Khire JM, Pant A (1992) Thermostable, salt-tolerant amylase from Bacillus sp. 64. World J Microbiol 8:167–170
Kobayashi T, Kamekura M, Kanlayakrit W, Onishi H (1986) Production, purification, and characterization of an amylase from the moderate halophilic Micrococcus varians subspecies halophilus. Microbios 46:165–177
Lin LL, Chyau CC, Hsu WH (1998) Production and properties of a raw starch-degrading amylase from the thermophilic and alkalophilic Bacillus sp. TS-23. Biotechnol Appl Biochem 28:61–68
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Mijts BN, Patel BK (2002) Cloning, sequencing and expression of an alpha-amylase gene, amyA, from the thermophilic halophile Halothermothrix orenii and purification and biochemical characterization of the recombinant enzyme. Microbiology 148:2343–2349
Mohapatra BR, Baberjee UC, Bapuji M (1998) Characterization of a fungal amylase from Mucor sp. associated with the marine sponge Spirastrella sp. J Biotechnol 60:113–117
Onishi H (1972) Halophilic amylase from a moderately halophilic Micrococcus. J Bacteriol 109:570–574
Onishi H, Hidaka H (1978) Purification and some properties of amylase produced by a moderately halophilic Acinetobacter sp. Can J Microbiol 24:1017–1023
Onishi H, Sonada K (1979) Purification and some properties of an extracellular amylase from moderate halophilic Micrococcus halobius. Appl Environ Microbiol 38:616–620
Rao MB, Tanksale AM, Gathe MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Sanchez-Porro C, Mellado E, Bertoldo C, Antranikian G, Ventosa A (2003) Screening and characterization of the protease CP1 produced by the moderately halophilic bacterium Pseudoalteromonas sp. Strain CP76. Extremophiles 7:221–228
Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A (2006) α-Amylases from microbial sources—an overview on recent developments. Food Technol Biotechnol 44:173–184
Srimathi S, Jayaraman G, Feller G, Danielsson B, Narayanan PR (2007) Intrinsic halotolerance of the psychrophilic alpha-amylase from Pseudoalteromonas haloplanktis. Extremophiles 11:505–515
Teodoro CED, Martin MLL (2000) Culture conditions for the production of thermostable amylase by Bacillus sp. Braz J Microbiol 31:298–302
Tian X-P, Dastager SG, Lee J-C, Tang S-K, Zhang Y-Q, Park D-J, Kim C-J, Li W-J (2007) Alkalibacillus halophilus sp. nov., a new halophilic species isolated from hypersaline soil in Xin-Jiang province, China. Syst Appl Microbiol 30:268–272
Ventosa A, Garcia MT, Kamekura M, Onishi H, Ruiz-Berraquero F (1989) Bacillus halophilus sp. Nov., a moderately halophilic Bacillus species. Syst Appl Microbiol 12:162–166
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Wejse PL, Ingvorsen K, Mortensen KK (2003) Xylanase production by a novel halophilic bacterium increased 20-fold by response surface methodology. Process Biochem 32:721–727
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanthi Kiran, K., Chandra, T.S. Production of surfactant and detergent-stable, halophilic, and alkalitolerant alpha-amylase by a moderately halophilic Bacillus sp. Strain TSCVKK . Appl Microbiol Biotechnol 77, 1023–1031 (2008). https://doi.org/10.1007/s00253-007-1250-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1250-z