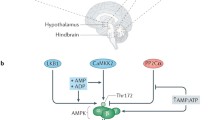
Abstract
Although obesity is an epidemic threat to general health worldwide, an effective treatment has yet to be found. Insights into weight-regulatory pathways will accelerate the identification of new molecular targets for anti-obesity agents. 5′-AMP-activated protein kinase (AMPK) is an enzyme activated during low cellular energy charge. In peripheral tissues, the activation of AMPK influences various metabolic pathways, including glucose uptake, glycolysis, and fatty acid oxidation, all of which help to re-establish a normal cellular energy balance. AMPK is also present in the neurons of the hypothalamus, a critical center in the regulation of energy homeostasis. Recent studies from our group and others have shown that many factors (α-lipoic acid, leptin, insulin, ghrelin, glucose, 2-deoxyglucose, etc.) cause an alteration in hypothalamic AMPK activity that mediates effects on feeding behavior. Hypothalamic AMPK also appears to play a role in the central regulation of energy expenditure and peripheral glucose metabolism. These data indicate that hypothalamic AMPK is an important signaling molecule that integrates nutritional and hormonal signals and modulates feeding behavior and energy metabolism.

Similar content being viewed by others
References
Flegal KM, Carroll MD, Kuczmarski RJ, et al (1998) Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 22:39–47
Gordon T, Kannel WB (1976) Obesity and cardiovascular diseases: the Framingham study. Clin Endocrinol Metab 5:367–375
Schwartz MW, Woods SC, Porte D Jr, et al (2000) Central nervous system control of food intake. Nature 404:661–671
Kemp BE, Stapleton D, Campbell DJ, et al (2003) AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31:162–168
Hardie DG, Salt IP, Hawley SA, et al (1999) AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J 338:717–722
Turnley AM, Stapleton D, Mann RJ, et al (1999) Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72:1707–1716
Culmsee C, Monnig J, Kemp BE, et al (2001) AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 17:45–58
Kim MS, Park JY, Namkoong C, et al (2004) Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10:727–733
Minokoshi Y, Alquier T, Furukawa N, et al (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574
Andersson U, Filipsson K, Abbott CR, et al (2004) AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008
Beg ZH, Allmann DW, Gibson DM (1973) Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and with protein fractions of rat liver cytosol. Biochem Biophys Res Commun 54:1362–1369
Carlson CA, Kim KH (1973) Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem 248:378–380
Yeh LA, Lee KH, Kim KH (1980) Regulation of rat liver acetyl-CoA carboxylase. Regulation of phosphorylation and inactivation of acetyl-CoA carboxylase by the adenylate energy charge. J Biol Chem 255:2308–2314
Hardie DG, Guy PS (1980) Reversible phosphorylation and inactivation of acetyl-CoA carboxylase from lactating rat mammary gland by cyclic AMP-dependent protein kinase. Eur J Biochem 110:167–177
Stapleton D, Woollatt E, Mitchelhill KI, et al (1997) AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Lett 409:452–456
da Silva Xavier G, Leclerc I, Salt IP, et al (2000) Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc Natl Acad Sci USA 97:4023–4028
Woods A, Azzout-Marniche D, Foretz M, et al (2000) Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol 20:6704–6711
Chen Z, Heierhorst J, Mann RJ, et al (1999) Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett 460:343–348
Hardie DG, Salt IP, Hawley SA, et al (1999) AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J 338:717–722
Vavvas D, Apazidis A, Saha AK, et al (1997) Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem 272:13255–13261
Christopher MJ, Chen ZP, Rantzau C, et al (2003) Skeletal muscle basal AMP-activated protein kinase activity is chronically elevated in alloxan-diabetic dogs: impact of exercise. J Appl Physiol 95:1523–1530
Marsin AS, Bouzin C, Bertrand L, et al (2002) The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem 277:30778–30783
Choi SL, Kim SJ, Lee KT, et al (2001) The regulation of AMP-activated protein kinase by H2O2. Biochem Biophys Res Commun 287:92–97
Hardie DG (1999) Roles of the AMP-activated/SNF1 protein kinase family in the response to cellular stress. Biochem Soc Symp 64:13–27
Patel N, Khayat ZA, Ruderman NB, et al (2001) Dissociation of 5′ AMP-activated protein kinase activation and glucose uptake stimulation by mitochondrial uncoupling and hyperosmolar stress: differential sensitivities to intracellular Ca2+ and protein kinase C inhibition. Biochem Biophys Res Commun 285:1066–1070
Zhou G, Myers R, Li Y, et al (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174
Mu J, Brozinick JT Jr, Valladares O, et al (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094
Goodyear LJ, Kahn BB (1998) Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49:235–261
Holmes BF, Lang DB, Birnbaum MJ, et al (2004) AMP kinase is not required for the GLUT4 response to exercise and denervation in skeletal muscle. Am J Physiol Endocrinol Metab 287:E739–E743
Ruderman NB, Saha AK, Kraegen EW (2003) Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology 144:5166–5171
Minokoshi Y, Kim YB, Peroni OD, et al (2002) Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415:339–343
Tomas E, Tsao TS, Saha AK, et al (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99:16309–16313
Yamauchi T, Kamon J, Minokoshi Y, et al (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295
Zong H, Ren JM, Young LH, et al (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 99:15983–15987
Fiedler M, Zierath JR, Selen G, et al (2001) 5-aminoimidazole-4-carboxy-amide-1-beta-D-ribofuranoside treatment ameliorates hyperglycaemia and hyperinsulinaemia but not dyslipidaemia in KKAy-CETP mice. Diabetologia 44:2180–2186
Leclerc I, Lenzner C, Gourdon L, et al (2001) Hepatocyte nuclear factor-4alpha involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes 50:1515–1521
Horman S, Browne G, Krause U, et al (2002) Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol 12:1419–1423
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Salt IP, Connell JM, Gould GW (2000) 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. Diabetes 49:1649–1656
Sullivan JE, Brocklehurst KJ, Marley AE, et al (1994) Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 353:33–36
Yin W, Mu J, Birnbaum MJ (2001) Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J Biol Chem 278:43074–43080
da Silva Xavier G, Leclerc I, Salt IP, et al (2000) Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc Natl Acad Sci USA 97:4023–4028
da Silva Xavier G, Leclerc I, Varadi A, et al (2003) Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 371:761–774
Kefas BA, Heimberg H, Vaulont S, et al (2003) AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 46:250–254
Unger RH, Zhou YT (2001) Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 50[Suppl 1]:S118–S121
Diraison F, Parton L, Ferre P, et al (2004) Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR). Biochem J 378:769–778
Davis JD, Wirtshafter D, Asin KE, et al (1981) Sustained intracerebroventricular infusion of brain fuels reduces body weight and food intake in rats. Science 212:81–83
Obici S, Feng Z, Morgan K, et al (2002) Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51:271–275
Thompson DA, Campbell RG (1977) Hunger in humans induced by 2-deoxy-D-glucose: glucoprivic control of taste preference and food intake. Science 198:1065–1068
Sergeyev V, Broberger C, Gorbatyuk O, et al (2000) Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport 11:117–121
Loftus TM, Jaworsky DE, Frehywot GL, et al (2000) Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288:2379–2381
Obici S, Feng Z, Arduini A, Conti R, et al (2003) Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9:756–761
Levin BE (2001) Glucosensing neurons do more than just sense glucose. Int J Obes Relat Metab Disord 25[Suppl 5]:S68–S72
Miki T, Liss B, Minami K, et al (2001) ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4:507–512
Spanswick D, Smith MA, Groppi VE, et al (1997) Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390:521–525
Spanswick D, Smith MA, Mirshamsi S, et al (2000) Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3:757–758
Kim EK, Miller I, Aja S, et al (2004) C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 279:19970–19976
Landree LE, Hanlon AL, Strong DW, et al (2004) C75, a fatty acid synthase inhibitor, modulates AMP-activated protein kinase to alter neuronal energy metabolism. J Biol Chem 279:3817–3827
Namkoong C, Kim MS, Jang PG, et al (2005) Enhanced hypothalamic AMP-activated protein kinase activity contributes to hyperphagia in diabetic rats. Diabetes 54:63–68
Lee K, Li B, Xi X, Suh Y, et al (2005) Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology 146:3–10
Light PE, Wallace CH, Dyck JR (2003) Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation 107:1962–1965
Hallows KR, Raghuram V, Kemp BE, et al (2000) Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105:1711–1721
Minokoshi Y, Kim YB, Peroni OD, et al (2002) Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415:339–343
Viollet B, Andreelli F, Jorgensen SB, et al (2003) The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111:91–98
Penicaud L, Leloup C, Lorsignol A, et al (2002) Brain glucose sensing mechanism and glucose homeostasis. Curr Opin Clin Nutr Metab Care 5:539–543
Minokoshi Y, Haque MS, Shimazu T (1999) Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48:287–291
Obici S, Zhang BB, Karkanias G, et al (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382
Obici S, Feng Z, Tan J, et al (2001) Central melanocortin receptors regulate insulin action. J Clin Invest 108:1079–1085
Perrin C, Knauf C, Burcelin R (2004) Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, controls muscle glycogen synthesis. Endocrinology 145:4025–4033
McCrimmon RJ, Fan X, Ding Y, et al (2004) Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes 53:1953–1958
Acknowledgements
This work was supported by grants from the Korean Ministry of Science and Technology (M1040000000804J000000810), Korean Ministry of Health and Welfare (03-PJ1-PG1-CH05-0005), and the Asan Institute of Life Sciences (04-326).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.S., Lee, K.U. Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J Mol Med 83, 514–520 (2005). https://doi.org/10.1007/s00109-005-0659-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-005-0659-z