Abstract
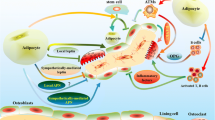
The number of mature osteoblasts and marrow adipocytes in bone is influenced by the differentiation of the common mesenchymal progenitor cell towards one phenotype and away from the other. Consequently, factors which promote adipogenesis not only lead to fatty marrow but also inhibit osteoblastogenesis, resulting in decreased osteoblast numbers, diminished bone formation and, potentially, inadequate bone mass and osteoporosis. In addition to osteoblast and bone adipocyte numbers being influenced by this skewing of progenitor cell differentiation towards one phenotype, mature osteoblasts and adipocytes secrete factors which may evoke changes in the cell fate and function of each other. This review examines the endogenous factors, such as PPAR-γ2, Wnt, IGF-1, GH, FGF-2, oestrogen, the GP130 signalling cytokines, vitamin D and glucocorticoids, which regulate the selection between osteoblastogenesis and adipogenesis and the interrelationship between fat and bone. The role of adipokines on bone, such as adiponectin and leptin, as well as adipose-derived oestrogen, is reviewed and the role of bone as an energy regulating endocrine organ is discussed.


Similar content being viewed by others
References
Gomez-Ambrosi J, Rodriguez A, Catalan V, Fruhbeck G (2008) The bone-adipose axis in obesity and weight loss. Obes Surg 18:1134–1143
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19:109–124
Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B (2009) PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 106:232–246
Gimble JM, Robinson CE, Wu X, Kelly KA (1996) The function of adipocytes in the bone marrow stroma: an update. Bone 19:421–428
Lecka-Czernik B (2010) PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Curr Osteoporos Rep 8:84–90
Lecka-Czernik B (2012) Marrow fat metabolism is linked to the systemic energy metabolism. Bone 50:534–539
Abdallah BM, Kassem M (2012) New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone 50:540–545
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B (2012) Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 50:546–552
Kawai M, Rosen CJ (2010) PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol 6:629–636
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171
Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55:693–698
Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V (2008) Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 93:2281–2286
Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB (2007) MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647
Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK (2011) Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 19:49–53
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ III, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Song L, Tuan RS (2004) Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J 18:980–982
Nuttall ME, Gimble JM (2004) Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol 4:290–294
Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME (2006) Playing with bone and fat. J Cell Biochem 98:251–266
Hasegawa T, Oizumi K, Yoshiko Y, Tanne K, Maeda N, Aubin JE (2008) The PPARgamma-selective ligand BRL-49653 differentially regulates the fate choices of rat calvaria versus rat bone marrow stromal cell populations. BMC Dev Biol 8:71
Schilling T, Noth U, Klein-Hitpass L, Jakob F, Schutze N (2007) Plasticity in adipogenesis and osteogenesis of human mesenchymal stem cells. Mol Cell Endocrinol 271:1–17
Piters E, Boudin E, Van Hul W (2008) Wnt signaling: a win for bone. Arch Biochem Biophys 473:112–116
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R (2000) Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24:391–395
Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312
Smink JJ, Leutz A (2012) Instruction of mesenchymal cell fate by the transcription factor C/EBPbeta. Gene 497:10–17
Dingwall M, Marchildon F, Gunanayagam A, Louis CS, Wiper-Bergeron N (2011) Retinoic acid-induced Smad3 expression is required for the induction of osteoblastogenesis of mesenchymal stem cells. Differentiation 82:57–65
Zuo Y, Qiang L, Farmer SR (2006) Activation of CCAAT/enhancer-binding protein (C/EBP) alpha expression by C/EBP beta during adipogenesis requires a peroxisome proliferator-activated receptor-gamma-associated repression of HDAC1 at the C/ebp alpha gene promoter. J Biol Chem 281:7960–7967
Sottile V, Seuwen K (2000) Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone). FEBS Lett 475:201–204
Pereira RC, Delany AM, Canalis E (2002) Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone 30:685–691
Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J, Ishii S (2006) Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell 10:461–471
Zehentner BK, Leser U, Burtscher H (2000) BMP-2 and sonic hedgehog have contrary effects on adipocyte-like differentiation of C3H10T1/2 cells. DNA Cell Biol 19:275–281
Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, Blanchet AM, Adelmant G, Baron R, Roman-Roman S (2001) Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci 114:2085–2094
Yuasa T, Kataoka H, Kinto N, Iwamoto M, Enomoto-Iwamoto M, Iemura S, Ueno N, Shibata Y, Kurosawa H, Yamaguchi A (2002) Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol 193:225–232
Salazar VS, Mbalaviele G, Civitelli R (2008) The pro-osteogenic action of beta-catenin requires interaction with BMP signaling, but not Tcf/Lef transcriptional activity. J Cell Biochem 104:942–952
Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK (1995) Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci USA 92:7921–7925
Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J (1997) The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 272:18779–18789
Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM (1994) mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8:1224–1234
Heikkinen S, Auwerx J, Argmann CA (2007) PPARgamma in human and mouse physiology. Biochim Biophys Acta 1771:999–1013
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H (2004) PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855
Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79:1147–1156
Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T (1999) PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4:597–609
Kawaguchi H, Akune T, Yamaguchi M, Ohba S, Ogata N, Chung UI, Kubota N, Terauchi Y, Kadowaki T, Nakamura K (2005) Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J Bone Miner Metab 23:275–279
Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL (1999) Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem 74:357–371
Liu LF, Shen WJ, Zhang ZH, Wang LJ, Kraemer FB (2010) Adipocytes decrease Runx2 expression in osteoblastic cells: roles of PPARgamma and adiponectin. J Cell Physiol 225:837–845
Sadie-Van Gijsen H, Crowther NJ, Hough FS, Ferris WF (2010) Depot-specific differences in the insulin response of adipose-derived stromal cells. Mol Cell Endocrinol 328:22–27
Medina-Gomez G, Gray S, Vidal-Puig A (2007) Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1). Public Health Nutr 10:1132–1137
Berger J, Patel HV, Woods J, Hayes NS, Parent SA, Clemas J, Leibowitz MD, Elbrecht A, Rachubinski RA, Capone JP, Moller DE (2000) A PPARgamma mutant serves as a dominant negative inhibitor of PPAR signaling and is localized in the nucleus. Mol Cell Endocrinol 162:57–67
Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R (2007) Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol 27:803–817
Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM (1994) Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res 22:5628–5634
Schoonjans K, Staels B, Auwerx J (1996) Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 37:907–925
Forman BM, Chen J, Evans RM (1996) The peroxisome proliferator-activated receptors: ligands and activators. Ann N Y Acad Sci 804:266–275
Hu E, Kim JB, Sarraf P, Spiegelman BM (1996) Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 274:2100–2103
Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S (2007) A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 9:1273–1285
Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL (2002) Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143:2376–2384
Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR (2005) Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology 146:3622–3631
Botolin S, McCabe LR (2006) Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol 209:967–976
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39
Krishnan V, Bryant HU, MacDougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209
Takada I, Kouzmenko AP, Kato S (2009) Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5:442–447
Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, MacDougald OA (2012) Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone 50:477–489
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102:3324–3329
Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, Rawadi G (2005) Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone 36:585–598
Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F (2005) Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132:49–60
Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA (2008) Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem 283:20505–20522
Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA (2003) MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem 278:45969–45977
Ichida F, Nishimura R, Hata K, Matsubara T, Ikeda F, Hisada K, Yatani H, Cao X, Komori T, Yamaguchi A, Yoneda T (2004) Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem 279:34015–34022
Sadie-Van Gijsen H, Smith W, du Toit EF, Michie J, Hough FS, Ferris WF (2012) Depot-specific and hypercaloric diet-induced effects on the osteoblast and adipocyte differentiation potential of adipose-derived stromal cells. Mol Cell Endocrinol 348:55–66
Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR (2003) Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J 376:607–613
Kulkarni NH, Onyia JE, Zeng Q, Tian X, Liu M, Halladay DL, Frolik CA, Engler T, Wei T, Kriauciunas A, Martin TJ, Sato M, Bryant HU, Ma YL (2006) Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res 21:910–920
Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G (2005) Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA 102:17406–17411
Li HX, Luo X, Liu RX, Yang YJ, Yang GS (2008) Roles of Wnt/beta-catenin signaling in adipogenic differentiation potential of adipose-derived mesenchymal stem cells. Mol Cell Endocrinol 291:116–124
Day TF, Guo X, Garrett-Beal L, Yang Y (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750
Kostenuik PJ, Shalhoub V (2001) Osteoprotegerin: a physiological and pharmacological inhibitor of bone resorption. Curr Pharm Des 7:613–635
Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Kassem M, Mosekilde L, Eriksen EF (1994) Growth hormone stimulates proliferation of normal human bone marrow stromal osteoblast precursor cells in vitro. Growth Regul 4:131–135
Nilsson A, Swolin D, Enerback S, Ohlsson C (1995) Expression of functional growth hormone receptors in cultured human osteoblast-like cells. J Clin Endocrinol Metab 80:3483–3488
Herington AC (1981) Identification and characterization of growth hormone receptors on isolated rat adipocytes. J Recept Res 2:299–316
Gevers EF, Loveridge N, Robinson IC (2002) Bone marrow adipocytes: a neglected target tissue for growth hormone. Endocrinology 143:4065–4073
Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M (2004) Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35:1046–1058
Schwander JC, Hauri C, Zapf J, Froesch ER (1983) Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology 113:297–305
Canalis E, McCarthy T, Centrella M (1988) Isolation and characterization of insulin-like growth factor I (somatomedin-C) from cultures of fetal rat calvariae. Endocrinology 122:22–27
Yakar S, Rosen CJ (2003) From mouse to man: redefining the role of insulin-like growth factor-I in the acquisition of bone mass. Exp Biol Med (Maywood) 228:245–252
Thomas T, Gori F, Spelsberg TC, Khosla S, Riggs BL, Conover CA (1999) Response of bipotential human marrow stromal cells to insulin-like growth factors: effect on binding protein production, proliferation, and commitment to osteoblasts and adipocytes. Endocrinology 140:5036–5044
Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL (2000) Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141:2674–2682
Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL (2002) Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277:44005–44012
Nicolas V, Prewett A, Bettica P, Mohan S, Finkelman RD, Baylink DJ, Farley JR (1994) Age-related decreases in insulin-like growth factor-I and transforming growth factor-beta in femoral cortical bone from both men and women: implications for bone loss with aging. J Clin Endocrinol Metab 78:1011–1016
Sugimoto T, Nishiyama K, Kuribayashi F, Chihara K (1997) Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res 12:1272–1279
Kawaguchi H (2006) Molecular backgrounds of age-related osteoporosis from mouse genetics approaches. Rev Endocr Metab Disord 7:17–22
Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP (1998) Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab 83:4257–4262
Garnero P, Sornay-Rendu E, Delmas PD (2000) Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 355:898–899
Lecka-Czernik B, Rosen CJ, Kawai M (2010) Skeletal aging and the adipocyte program: new insights from an “old” molecule. Cell Cycle 9:3648–3654
Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ (2007) Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology 148:903–911
Kawai M, Delany AM, Green CB, Adamo ML, Rosen CJ (2010) Nocturnin suppresses igf1 expression in bone by targeting the 3′ untranslated region of igf1 mRNA. Endocrinology 151:4861–4870
Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T (2002) Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 143:2349–2356
Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S (2008) Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int 19:1323–1330
Benayahu D, Shur I, Ben Eliyahu S (2000) Hormonal changes affect the bone and bone marrow cells in a rat model. J Cell Biochem 79:407–415
Elbaz A, Rivas D, Duque G (2009) Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology 10:747–755
Somjen D, Katzburg S, Kohen F, Gayer B, Posner GH, Yoles I, Livne E (2011) The effects of native and synthetic estrogenic compounds as well as vitamin D less-calcemic analogs on adipocytes content in rat bone marrow. J Endocrinol Invest 34:106–110
Kim S, Bi X, Czarny-Ratajczak M, Dai J, Welsh DA, Myers L, Welsch MA, Cherry KE, Arnold J, Poon LW, Jazwinski SM (2012) Telomere maintenance genes SIRT1 and XRCC6 impact age-related decline in telomere length but only SIRT1 is associated with human longevity. Biogerontology 13:119–131
Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado DO, Leid M, McBurney MW, Guarente L (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771–776
Backesjo CM, Li Y, Lindgren U, Haldosen LA (2006) Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res 21:993–1002
Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML, Yen ML (2011) Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res 26:2552–2563
Modder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S (2011) Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone 49:202–207
Modder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S (2011) Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res 26:27–34
Brunner G, Gabrilove J, Rifkin DB, Wilson EL (1991) Phospholipase C release of basic fibroblast growth factor from human bone marrow cultures as a biologically active complex with a phosphatidylinositol-anchored heparan sulfate proteoglycan. J Cell Biol 114:1275–1283
Sabbieti MG, Marchetti L, Abreu C, Montero A, Hand AR, Raisz LG, Hurley MM (1999) Prostaglandins regulate the expression of fibroblast growth factor-2 in bone. Endocrinology 140:434–444
Globus RK, Plouet J, Gospodarowicz D (1989) Cultured bovine bone cells synthesize basic fibroblast growth factor and store it in their extracellular matrix. Endocrinology 124:1539–1547
Slater M, Patava J, Kingham K, Mason RS (1994) Modulation of growth factor incorporation into ECM of human osteoblast-like cells in vitro by 17 beta-estradiol. Am J Physiol 267:E990–E1001
Itoh N, Ornitz DM (2004) Evolution of the Fgf and Fgfr gene families. Trends Genet 20:563–569
Locklin RM, Williamson MC, Beresford JN, Triffitt JT, Owen ME (1995) In vitro effects of growth factors and dexamethasone on rat marrow stromal cells. Clin Orthop Relat Res 313:27–35
Long MW, Robinson JA, Ashcraft EA, Mann KG (1995) Regulation of human bone marrow-derived osteoprogenitor cells by osteogenic growth factors. J Clin Invest 95:881–887
Naganawa T, Xiao L, Abogunde E, Sobue T, Kalajzic I, Sabbieti M, Agas D, Hurley MM (2006) In vivo and in vitro comparison of the effects of FGF-2 null and haplo-insufficiency on bone formation in mice. Biochem Biophys Res Commun 339:490–498
Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, Hurley MM (2010) Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone 47:360–370
Bravo J, Heath JK (2000) Receptor recognition by gp130 cytokines. EMBO J 19:2399–2411
Sims NA, Walsh NC (2010) GP130 cytokines and bone remodelling in health and disease. BMB Rep 43:513–523
Bellido T, Stahl N, Farruggella TJ, Borba V, Yancopoulos GD, Manolagas SC (1996) Detection of receptors for interleukin-6, interleukin-11, leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor in bone marrow stromal/osteoblastic cells. J Clin Invest 97:431–437
Kawashima I, Takiguchi Y (1992) Interleukin-11: a novel stroma-derived cytokine. Prog Growth Factor Res 4:191–206
Keller DC, Du XX, Srour EF, Hoffman R, Williams DA (1993) Interleukin-11 inhibits adipogenesis and stimulates myelopoiesis in human long-term marrow cultures. Blood 82:1428–1435
Okazaki R, Toriumi M, Fukumoto S, Miyamoto M, Fujita T, Tanaka K, Takeuchi Y (1999) Thiazolidinediones inhibit osteoclast-like cell formation and bone resorption in vitro. Endocrinology 140:5060–5065
Takeuchi Y, Watanabe S, Ishii G, Takeda S, Nakayama K, Fukumoto S, Kaneta Y, Inoue D, Matsumoto T, Harigaya K, Fujita T (2002) Interleukin-11 as a stimulatory factor for bone formation prevents bone loss with advancing age in mice. J Biol Chem 277:49011–49018
Kido S, Kuriwaka-Kido R, Imamura T, Ito Y, Inoue D, Matsumoto T (2009) Mechanical stress induces interleukin-11 expression to stimulate osteoblast differentiation. Bone 45:1125–1132
Tohjima E, Inoue D, Yamamoto N, Kido S, Ito Y, Kato S, Takeuchi Y, Fukumoto S, Matsumoto T (2003) Decreased AP-1 activity and interleukin-11 expression by bone marrow stromal cells may be associated with impaired bone formation in aged mice. J Bone Miner Res 18:1461–1470
Kodama Y, Takeuchi Y, Suzawa M, Fukumoto S, Murayama H, Yamato H, Fujita T, Kurokawa T, Matsumoto T (1998) Reduced expression of interleukin-11 in bone marrow stromal cells of senescence-accelerated mice (SAMP6): relationship to osteopenia with enhanced adipogenesis. J Bone Miner Res 13:1370–1377
Walker EC, McGregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JM, Gillespie MT, Martin TJ, Sims NA (2008) Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res 23:2025–2032
Quach JM, Walker EC, Allan E, Solano M, Yokoyama A, Kato S, Sims NA, Gillespie MT, Martin TJ (2011) Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. J Biol Chem 286:4186–4198
Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ, Constable MJ, Nicholson GC, Zhang JG, Nicola NA, Gillespie MT, Martin TJ, Sims NA (2010) Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest 120:582–592
Metcalf D, Gearing DP (1989) Fatal syndrome in mice engrafted with cells producing high levels of the leukemia inhibitory factor. Proc Natl Acad Sci USA 86:5948–5952
Metcalf D, Gearing DP (1989) A myelosclerotic syndrome in mice engrafted with cells producing high levels of leukemia inhibitory factor (LIF). Leukemia 3:847–852
Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini L, Villageois P, Barhanin B, Vernallis A, Smith AG, Ailhaud G, Dani C (1999) Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem 274:24965–24972
Falconi D, Oizumi K, Aubin JE (2007) Leukemia inhibitory factor influences the fate choice of mesenchymal progenitor cells. Stem Cells 25:305–312
Malaval L, Aubin JE (2001) Biphasic effects of leukemia inhibitory factor on osteoblastic differentiation. J Cell Biochem Suppl 36:63–70
Syed FA, Ng AC (2010) The pathophysiology of the aging skeleton. Curr Osteoporos Rep 8:235–240
Duque G, Macoritto M, Kremer R (2004) 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2). Exp Gerontol 39:333–338
Duque G, Macoritto M, Kremer R (2004) Vitamin D treatment of senescence accelerated mice (SAM-P/6) induces several regulators of stromal cell plasticity. Biogerontology 5:421–429
Duque G, Macoritto M, Dion N, Ste-Marie LG, Kremer R (2005) 1,25(OH)2D3 acts as a bone-forming agent in the hormone-independent senescence-accelerated mouse (SAM-P/6). Am J Physiol Endocrinol Metab 288:E723–E730
Wood RJ (2008) Vitamin D and adipogenesis: new molecular insights. Nutr Rev 66:40–46
Cianferotti L, Demay MB (2007) VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J Cell Biochem 101:80–88
Shalhoub V, Conlon D, Tassinari M, Quinn C, Partridge N, Stein GS, Lian JB (1992) Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J Cell Biochem 50:425–440
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328
Vande Berg BC, Malghem J, Lecouvet FE, Devogelaer JP, Maldague B, Houssiau FA (1999) Fat conversion of femoral marrow in glucocorticoid-treated patients: a cross-sectional and longitudinal study with magnetic resonance imaging. Arthritis Rheum 42:1405–1411
Rosen CJ, Klibanski A (2009) Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 122:409–414
Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, Rosen CJ, Gordon CM (2010) Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res 25:298–304
Fernandez-Rodriguez E, Stewart PM, Cooper MS (2009) The pituitary–adrenal axis and body composition. Pituitary 12:105–115
Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ (2008) Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem 283:1936–1945
Zhou H, Mak W, Kalak R, Street J, Fong-Yee C, Zheng Y, Dunstan CR, Seibel MJ (2009) Glucocorticoid-dependent Wnt signaling by mature osteoblasts is a key regulator of cranial skeletal development in mice. Development 136:427–436
Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R (2005) Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun 329:177–181
Kim CH, Cheng SL, Kim GS (1999) Effects of dexamethasone on proliferation, activity, and cytokine secretion of normal human bone marrow stromal cells: possible mechanisms of glucocorticoid-induced bone loss. J Endocrinol 162:371–379
Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schutz G, Tuckermann JP (2010) Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 11:517–531
Giustina A, Doga M, Bodini C, Girelli A, Legati F, Bossoni S, Romanelli G (1990) Acute effects of cortisone acetate on growth hormone response to growth hormone-releasing hormone in normal adult subjects. Acta Endocrinol (Copenh) 122:206–210
Delany AM, Durant D, Canalis E (2001) Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol 15:1781–1789
Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL (2002) Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA 99:9445–9449
Ali AT, Penny CB, Paiker JE, van Niekerk C, Smit A, Ferris WF, Crowther NJ (2005) Alkaline phosphatase is involved in the control of adipogenesis in the murine preadipocyte cell line, 3T3-L1. Clin Chim Acta 354:101–109
Ali AT, Penny CB, Paiker JE, Psaras G, Ikram F, Crowther NJ (2006) The relationship between alkaline phosphatase activity and intracellular lipid accumulation in murine 3T3-L1 cells and human preadipocytes. Anal Biochem 354:247–254
Liang J, Fu M, Ciociola E, Chandalia M, Abate N (2007) Role of ENPP1 on adipocyte maturation. PLoS One 2:e882
Weiss MJ, Cole DE, Ray K, Whyte MP, Lafferty MA, Mulivor RA, Harris H (1988) A missense mutation in the human liver/bone/kidney alkaline phosphatase gene causing a lethal form of hypophosphatasia. Proc Natl Acad Sci USA 85:7666–7669
Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R (2010) Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet 86:273–278
Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM (2010) Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet 86:267–272
Meyre D, Bouatia-Naji N, Tounian A, Samson C, Lecoeur C, Vatin V, Ghoussaini M, Wachter C, Hercberg S, Charpentier G, Patsch W, Pattou F, Charles MA, Tounian P, Clement K, Jouret B, Weill J, Maddux BA, Goldfine ID, Walley A, Boutin P, Dina C, Froguel P (2005) Variants of ENPP1 are associated with childhood and adult obesity and increase the risk of glucose intolerance and type 2 diabetes. Nat Genet 37:863–867
Korostishevsky M, Cohen Z, Malkin I, Ermakov S, Yarenchuk O, Livshits G (2010) Morphological and biochemical features of obesity are associated with mineralization genes’ polymorphisms. Int J Obes (Lond) 34:1308–1318
Krauss RM, Herbert PN, Levy RI, Fredrickson DS (1973) Further observations on the activation and inhibition of lipoprotein lipase by apolipoproteins. Circ Res 33:403–411
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31:547–555
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23:17–29
Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X (2006) Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr 83:146–154
Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW (2007) Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 92:1640–1646
Castro JP, Joseph LA, Shin JJ, Arora SK, Nicasio J, Shatzkes J, Raklyar I, Erlikh I, Pantone V, Bahtiyar G, Chandler L, Pabon L, Choudhry S, Ghadiri N, Gosukonda P, Muniyappa R, von Gicyzki H, McFarlane SI (2005) Differential effect of obesity on bone mineral density in White, Hispanic and African American women: a cross sectional study. Nutr Metab (Lond) 2:9
Afghani A, Goran MI (2006) Racial differences in the association of subcutaneous and visceral fat on bone mineral content in prepubertal children. Calcif Tissue Int 79:383–388
Taaffe DR, Villa ML, Holloway L, Marcus R (2000) Bone mineral density in older non-Hispanic Caucasian and Mexican-American women: relationship to lean and fat mass. Ann Hum Biol 27:331–344
Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE, Grunfeld C (2012) Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab 97:1337–1346
Holecki M, Wiecek A (2010) Relationship between body fat mass and bone metabolism. Pol Arch Med Wewn 120:361–367
Afghani A, Goran MI (2009) The interrelationships between abdominal adiposity, leptin and bone mineral content in overweight Latino children. Horm Res 72:82–87
Pollock NK, Bernard PJ, Wenger K, Misra S, Gower BA, Allison JD, Zhu H, Davis CL (2010) Lower bone mass in prepubertal overweight children with prediabetes. J Bone Miner Res 25:2760–2769
Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M (2010) Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab 95:1247–1255
Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y (2011) Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr 158:727–734
Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD (2009) Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94:3387–3393
Choi HS, Kim KJ, Kim KM, Hur NW, Rhee Y, Han DS, Lee EJ, Lim SK (2010) Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif Tissue Int 87:218–225
Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Harrington LM, Breggia A, Rosen CJ, Miller KK (2011) Determinants of bone mineral density in obese premenopausal women. Bone 48:748–754
Katzmarzyk PT, Barreira TV, Harrington DM, Staiano AE, Heymsfield SB, Gimble JM (2012) Relationship between abdominal fat and bone mineral density in white and African American adults. Bone 50:576–579
Beauregard C, Utz AL, Schaub AE, Nachtigall L, Biller BM, Miller KK, Klibanski A (2008) Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab 93:2063–2071
Kuk JL, Saunders TJ, Davidson LE, Ross R (2009) Age-related changes in total and regional fat distribution. Ageing Res Rev 8:339–348
Boskey AL, Coleman R (2010) Aging and bone. J Dent Res 89:1333–1348
Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP (1981) Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest 67:1361–1369
Bennett AE, Wahner HW, Riggs BL, Hintz RL (1984) Insulin-like growth factors I and II: aging and bone density in women. J Clin Endocrinol Metab 59:701–704
Lecka-Czernik B (2008) Local and systemic functions of bone fat and its contribution to the energy metabolism—the effect of diabetes and obesity on bone. J Musculoskelet Neuronal Interact 8:346–347
Vermeulen A (1976) The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab 42:247–253
Grodin JM, Siiteri PK, MacDonald PC (1973) Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab 36:207–214
Forney JP, Milewich L, Chen GT, Garlock JL, Schwarz BE, Edman CD, MacDonald PC (1981) Aromatization of androstenedione to estrone by human adipose tissue in vitro. Correlation with adipose tissue mass, age, and endometrial neoplasia. J Clin Endocrinol Metab 53:192–199
Frisch RE, Canick JA, Tulchinsky D (1980) Human fatty marrow aromatizes androgen to estrogen. J Clin Endocrinol Metab 51:394–396
Nawata H, Tanaka S, Tanaka S, Takayanagi R, Sakai Y, Yanase T, Ikuyama S, Haji M (1995) Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol 53:165–174
Suzuki N, Yano T, Nakazawa N, Yoshikawa H, Taketani Y (1995) A possible role of estrone produced in adipose tissues in modulating postmenopausal bone density. Maturitas 22:9–12
Haffner SM, Bauer RL (1992) Excess androgenicity only partially explains the relationship between obesity and bone density in premenopausal women. Int J Obes Relat Metab Disord 16:869–874
MacDonald PC, Edman CD, Hemsell DL, Porter JC, Siiteri PK (1978) Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol 130:448–455
Cleland WH, Mendelson CR, Simpson ER (1985) Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab 60:174–177
Ackerman GE, Smith ME, Mendelson CR, MacDonald PC, Simpson ER (1981) Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab 53:412–417
Hemsell DL, Grodin JM, Brenner PF, Siiteri PK, MacDonald PC (1974) Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. J Clin Endocrinol Metab 38:476–479
Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221:286–289
Garaulet M, Hernandez-Morante JJ, de Heredia FP, Tebar FJ (2007) Adiponectin, the controversial hormone. Public Health Nutr 10:1145–1150
Hu E, Liang P, Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35:842–849
Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, Ikeda T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H (2006) Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem 99:196–208
Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY (2006) Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res 21:1648–1656
Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, Reid IR, Cornish J (2009) In vitro and in vivo effects of adiponectin on bone. Endocrinology 150:3603–3610
Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY (2008) Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta 387:31–35
Jurimae J, Rembel K, Jurimae T, Rehand M (2005) Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res 37:297–302
Richards JB, Valdes AM, Burling K, Perks UC, Spector TD (2007) Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab 92:1517–1523
Iacobellis G, Iorio M, Napoli N, Cotesta D, Zinnamosca L, Marinelli C, Petramala L, Minisola S, D’Erasmo E, Letizia C (2011) Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J Endocrinol Invest 34:e12–e15
Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW (2003) Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33:646–651
Sucunza N, Barahona MJ, Resmini E, Fernandez-Real JM, Ricart W, Farrerons J, Rodriguez EJ, Marin AM, Puig T, Webb SM (2009) A link between bone mineral density and serum adiponectin and visfatin levels in acromegaly. J Clin Endocrinol Metab 94:3889–3896
Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS (1995) Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 96:1658–1663
MacDougald OA, Hwang CS, Fan H, Lane MD (1995) Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci USA 92:9034–9037
Enjuanes A, Supervia A, Nogues X, Diez-Perez A (2002) Leptin receptor (OB-R) gene expression in human primary osteoblasts: confirmation. J Bone Miner Res 17:1135
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Reid IR (2004) Leptin deficiency—lessons in regional differences in the regulation of bone mass. Bone 34:369–371
Hamrick MW, Pennington C, Newton D, Xie D, Isales C (2004) Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383
Williams GA, Callon KE, Watson M, Costa JL, Ding Y, Dickinson M, Wang Y, Naot D, Reid IR, Cornish J (2011) Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res 26:1698–1709
Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL (1999) Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140:1630–1638
Motyl KJ, McCabe LR (2009) Leptin treatment prevents type I diabetic marrow adiposity but not bone loss in mice. J Cell Physiol 218:376–384
Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA (2005) Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res 20:994–1001
Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG Jr, Karsenty G (2008) Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci USA 105:20529–20533
Confavreux CB, Levine RL, Karsenty G (2009) A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 310:21–29
Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G (2009) A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989
Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T (2007) Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology 148:3419–3425
Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G (2004) Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA 101:3258–3263
Thomas T, Burguera B, Melton LJ III, Atkinson EJ, O’Fallon WM, Riggs BL, Khosla S (2001) Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone 29:114–120
Blain H, Vuillemin A, Guillemin F, Durant R, Hanesse B, de Talance N, Doucet B, Jeandel C (2002) Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab 87:1030–1035
Zhang H, Xie H, Zhao Q, Xie GQ, Wu XP, Liao EY, Luo XH (2010) Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post-menopausal Chinese women. J Endocrinol Invest 33:707–711
Ozata M, Ozdemir IC, Licinio J (1999) Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84:3686–3695
Lee DE, Kehlenbrink S, Lee H, Hawkins M, Yudkin JS (2009) Getting the message across: mechanisms of physiological cross talk by adipose tissue. Am J Physiol Endocrinol Metab 296:E1210–E1229
Thommesen L, Stunes AK, Monjo M, Grosvik K, Tamburstuen MV, Kjobli E, Lyngstadaas SP, Reseland JE, Syversen U (2006) Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem 99:824–834
Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, Zhou HD, Wu XP, Liao EY (2007) Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int 80:201–210
Li W, Yu B, Li M, Sun D, Hu Y, Zhao M, Cui CB, Hou S (2010) NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochem Biophys Res Commun 391:1228–1233
Tomomatsu N, Aoki K, Alles N, Soysa NS, Hussain A, Nakachi H, Kita S, Shimokawa H, Ohya K, Amagasa T (2009) LPS-induced inhibition of osteogenesis is TNF-alpha dependent in a murine tooth extraction model. J Bone Miner Res 24:1770–1781
Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A, Auwerx J (2003) Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci USA 100:14457–14462
Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J (2004) Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep 5:1007–1012
Luther J, Driessler F, Megges M, Hess A, Herbort B, Mandic V, Zaiss MM, Reichardt A, Zech C, Tuckermann JP, Calkhoven CF, Wagner EF, Schett G, David JP (2011) Elevated Fra-1 expression causes severe lipodystrophy. J Cell Sci 124:1465–1476
Garg A (2011) Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab 96:3313–3325
Fleckenstein JL, Garg A, Bonte FJ, Vuitch MF, Peshock RM (1992) The skeleton in congenital, generalized lipodystrophy: evaluation using whole-body radiographic surveys, magnetic resonance imaging and technetium-99 m bone scintigraphy. Skeletal Radiol 21:381–386
Shinya T, Sato S, Akaki S, Ogata T, Kato K, Tone A, Kanazawa S (2007) Computed tomography findings of congenital generalized lipodystrophy: multiple nodular fatty liver and diffuse sclerosis of bones. Radiat Med 25:484–487
Bandeira FF, Miranda CR, Waechter C, Bandeira ME (2007) High bone mass associated with berardinelli lipodystrophy. Endocr Pract 13:764–769
Ducy P (2011) The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54:1291–1297
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Hauschka PV, Lian JB, Cole DE, Gundberg CM (1989) Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev 69:990–1047
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382:448–452
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, Clemens TL (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319
Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308
Jequier E (2002) Leptin signaling, adiposity, and energy balance. Ann NY Acad Sci 967:379–388
Prats-Puig A, Mas-Parareda M, Riera-Perez E, Gonzalez-Forcadell D, Mier C, Mallol-Guisset M, Diaz M, Bassols J, de Zegher F, Ibanez L, Lopez-Bermejo A (2010) Carboxylation of osteocalcin affects its association with metabolic parameters in healthy children. Diabetes Care 33:661–663
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T (2011) Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 22:187–194
Hwang YC, Jeong IK, Ahn KJ, Chung HY (2009) The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev 25:768–772
Acknowledgments
H. Sadie-Van Gijsen and W.F. Ferris were supported by the Medical Research Council (MRC) of South Africa. N.J. Crowther was supported by the National Health Laboratory Services (NHLS) of South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadie-Van Gijsen, H., Crowther, N.J., Hough, F.S. et al. The interrelationship between bone and fat: from cellular see-saw to endocrine reciprocity. Cell. Mol. Life Sci. 70, 2331–2349 (2013). https://doi.org/10.1007/s00018-012-1211-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1211-2