Abstract
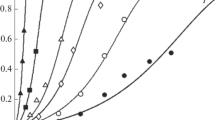
In this paper we report the point of zero charge (pHzpc) of natural magnetite and its adsorptive properties with regard to alkali metal ions. It has been found that pHzpc of freshly ground, untreated magnetite is 6.5, decreasing, after treatment with HCl, to the value of pH 3.8. This discrepancy might be explained as a consequence of possible structure changes on the magnetite surface. Adsorption properties of magnetite with respect to Li+, Na+ and K+ ions in the concentration and pH ranges varying from 0.1 to 1.0 mol·dm−3 and 6.5 to 10, respectively, were examined. For the used chloride media used, the adsorption sequence obtained is Na+>K+>Li+.
Similar content being viewed by others
References
P. V. BALAKRISHNAN, Canadian J. Chem. Eng., 55 (1977) 357.
C. F. BAES, Jr., T. H. HANDLEY, US AEC Document ORNL-3238, 1961.
C. F. BAES, Jr., T. H. HANDLEY, US AEC Document ORNL-3416, 1962.
C. F. BAES, Jr., T. H. HANDLEY, US AEC Document TID-7641, 1962.
F. H. SWEETON, C. F. BAES, Jr., R. W. RAY, US AEC Document ORNL-3789, 1965.
F. H. SWEETON, C. F. BAES, Jr., R. W. RAY, T. H. HANDLEY, US AEC Document ORNL-3591, 1964.
C. F. PAULSON, Chem. Eng. Progr., 56 (1964) 64.
P. J. ANDERSON, Atomic Energy Research Estab., M/R 2046, 1956.
S. M. AHMED, D. MAKSIMOV, Mines Branch Research Report, R 196, Dept. of Energy, Mines and Resources, Ottawa, Canada, 1968.
S. M. AHMED, D. MAKSIMOV, Can. J. Chem., 46 (1968) 3841.
I. IWASAKI, S. R. B. COOKE, Y. S. KIM, Trans. Am. Inst. Min. Engrs., 223 (1962) 113.
S. M. AHMED, J. Phys. Chem., 73 (1969) 3546.
Dj. M. PETKOVIĆ, S. K. MILONJIĆ, Bull. Inst. Nucl. Sci. “Boris Kidric”, 20 (1969) 499.
S. K. MILONJIĆ, A. RUVARAC, Bull. Inst. Nucl. Sci. “Boris Kidric”., 21 (1970) 462.
P. H. TEWARI, A. W. MCLEAN, J. Colloid Interface Sci., 40 (1972) 267.
P. H. TEWARI, A. B. CAMPBELL, W. LEE, Can. J. Chem., 50 (1972) 1642.
S. K. MILONJIĆ, M. Sc. Thesis, University of Belgrade 1973.
D. P. BENTON, G. A. HORSFALL, J. Chem. Soc., (1962) 3899.
J. LASKOWSKI, S. SOBIEROJ, Trans. Inst. Mining Met., Sect. C. 78 (1969) 161.
J. SHIMOIZAKA, Tohoku Kozan, 6 (1959) 99.
S. FUJIGAKI, A. IKEHATA, Y. KUMAGAI, K. NAKAGAVA, Kogyo Yosni, 108 (1967) 22.
H. SCHNEIDER, Gas-Wasserfach, Wasser-Abwasser, 111 (1970) 21.
P. H. TEWARI, R. H. TUXWORTH, W. LEE, Proc. Symp. Oxide-Electrolyte Interfaces, Electrochem. Soc., 1973, p. 91.
S. K. MILONJIĆ, A. LJ. RUVARAC, M. V. ŠUŠIĆ, Termochim. Acta, 11 (1975) 261. There is an error in this paper, i. e. it was indicated that Fe3O4 was treated with 2 mol·dm−3 HNO3. Actually, magnetite was washed with distilled water only, as it is originally indicated in Ref.17.
E. A. NECHAEV, V. A. VOLGINA, Dep. VINITI (USSR), No. 3067-76, Dep. Aug. 10, 1976.
S. K. MILONJIĆ, A. LJ, RUVARAC, M. V. ŠUŠIČ, Bull. Soc. Chim. Beograd, 43 (1978) 207.
B. VANKATARAMANI, K. S. VENKATERSWALU, J. SHANKAR, L. H. BEATSLE, Proc. Indian Acad. Sci., 87 (1978) 415.
B. VANKATARAMANI, K. S. VENKATESWALU, J. SHANKAR, J. Colloid Interface Sci., 67 (1978) 187.
S. M. AHMED in Oxide and Oxide Films, J. W. DIGGLE (Ed.), Vol. 2, Marcel Dekker, New York, 1973, p. 319–517.
G. A. PARKS, P. L. de BRUYN, J. Phys. Chem., 66 (1962) 967.
See for example: D. E. YATES, T. W. HEALY, J. C. S. Faraday I, 76 (1980) 9.
T. MORIMOTO, S. KITTAKA, Bull. Chem. Soc. Japan, 46 (1973) 3040.
S. KITTAKA, J. Colloid Interface Sci., 48 (1974) 327.
P. J. ANDERSON, Proc. 2nd Intern. Cong. Surface Activity, London, 3 (1957) 67. From “Recent Progress in Surface Science”., J. F. DANIELLI, K. G. A. PANKHURST, A. C. RIDDIFORD, (Eds.), Vol. 2, Academic Press, New York, 1964, p. 189.
YU. M. CHERNOBERZHSKII, V. I. DERDULLA, Elektropoverkhnostnye yavleniya v dispersnykh sistemakh, Nauka, Moskva, 1972, p. 24–37.
J. LYKLEMA, Croat. Chim. Acta, 43 (1971) 249.
J. LYKLEMA, T. W. HEALY, Disc. Faraday. Soc., 52 (1971) 318.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Milonjić, S.K., Kopečni, M.M. & Ilić, Z.E. The point of zero charge and adsorption properties of natural magnetite. J. Radioanal. Chem. 78, 15–24 (1983). https://doi.org/10.1007/BF02519745
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02519745