Summary
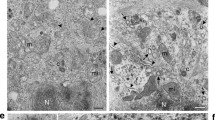
West Nile (Sarafend) virus was distinctly observed to bud from the plasma membrane rather than mature intracellularly. This has been observed with transmission electron microscopy. Using conventional scanning electron microscopy, budding at the plasma membrane especially at the filopodia was clearly illustrated. Immunogold labelling against the virus envelope protein was also performed to confirm this mode of exit. The gold particles were observed to be located at the sites where virus budding was seen under the field emission scanning electron microscope.
Similar content being viewed by others
References
Cardiff RD, Lund JK (1976) Distribution of dengue-2 antigens by electron immunochemistry. Infect Immun 13: 1699–1790
Castle E, Nowak T, Leidner U, Wengler G, Wengler G (1985) Sequence analysis of the viral core protein and the membrane-associated proteins V1 and NV2 of the flavivirus West Nile virus and of the genome sequence of these proteins. Virology 145: 227–236
Catanzaro PJ, Brandt WE, Hogrefe WR, Russell PK (1974) Detection of dengue cell-surface antigens by peroxidase-labelled antibodies and immune cytolysis. Infect Immun 10: 381–388
Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y (1988) Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem 263: 18545–18552
George S, Gourie-Devi M, Rao JA, Prasad SR, Pravi KM (1984) Isolation of West Nile virus from the brains of children who have died of encephalitis. Bull World Health Organ 62: 879–882
Hase T, Summers PL, Eckels KH, Baze WB (1987a) An electron and immunoelectron microscopic study of dengue-2 virus infection of cultured mosquito cells: maturation events. Arch Virol 92: 273–291
Hase T, Summers PL, Eckels KH, Baze WB (1987b) Maturation process of Japanese encephalitis virus in cultured mosquito cells in vitro and mouse brain cells in vivo. Arch Virol 96: 135–151
Ho ESP, Somasundram C, Ng ML (1987) Comparative ultrastructural studies of the three flaviviruses in Vero cells. Trop Med 29: 65–79
Ishak R, Tovey DG, Howard CR (1988) Morphogenesis of yellow fever virus 17D in infected cell culture. J Gen Virol 69: 325–335
Ko KK, Igarashi A, Fukai K (1979) Electron microscopic observation onAedes albopictus cells infected with dengue viruses. Arch Virol 62: 41–52
Leary K, Blair CD (1980) Sequential events in the morphogenesis of Japanese encephalitis virus. J Ultrastruct Res 72: 123–129
Matsumura T, Shiraki K, Sashitaka T, Hotta S (1977) Morphogenesis of dengue-1 virus in cultures of a human leukemic leukocyte line (J-111). Microbiol Immunol 21: 329–334
Murphy FA (1980) Togavirus morphology and morphogenesis. In: Schlesinger RW (ed) The togaviruses. Biology, structure, replication. Academic Press, New York, pp 241–316
Ng ML (1987) Ultrastructural studies of kunjin virus-infectedAedes albopictus cells. J Gen Virol 68: 577–582
Ng ML, Corner LC (1989) Detection of some dengue-2 virus antigens in infected cells using immunomicroscopy. Arch Virol 104: 197–208
Ng ML, Choo WKP, Ho YL (1992) Detection of flavivirus antigens in purified infected Vero cell plasma membrane J Virol Methods 39: 125–138
Ota Z (1965) Electron microscopic study of the development of Japanese B encephalitis virus in porcine kidney stable (PS) cells. Virology 25: 372–378
Sreenivasan V, Ng KL, Ng ML (1993) Brefeldin A affects West Nile virus replication in Vero cells but not C6/36 cells. J Virol Methods 45: 1–17
Sriurairatna S, Bhamarapravati N (1977) Replication of dengue-2 virus inAedes albopictus. Am J Trop Med Hyg 26: 1199–1205
Stohlman SA, Wisseman CL, Eylar OR, Silverman DJ (1975) Dengue virus-induced modifications of host cell membranes. J Virol 16: 1017–1026
Westaway EG (1973) Proteins specified by group B togaviruses in mammalian cells during productive infections. Virology 51: 454–465
Westaway EG, Ng ML (1980) Replication of flavivirus. Separation of membrane translation sites of kunjin virus protein and of cell protein. Virology 106: 107–122
Whealy ME, Card JP, Meade RP, Robbins AK, Enquist LW (1991) Effect of Brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol 65: 1066–1081
Wright PJ (1982) Envelope protein of the flavivirus Kunjin is apparently not glycosylated. J Gen Virol 59: 29–38
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ng, M.L., Howe, J., Sreenivasan, V. et al. Flavivirus West Nile (Sarafend) egress at the plasma membrane. Archives of Virology 137, 303–313 (1994). https://doi.org/10.1007/BF01309477
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309477