Abstract
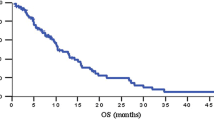
Gemcitabine is a novel nucleoside analog which demonstrated a broad spectrum of preclinical acitivity in solid tumor models, and responses in patients with pancreas cancer during phase I evaluation. Patients with measurable adenocarcinoma of the pancreas who had received no previous chemotherapy were eligible for this multicenter phase II clinical trial. Gemcitabine 800 mg/m2 was administered intravenously weekly for 3 consecutive weeks, followed by one week rest, every 4 weeks. Forty-four patients entered the trial; 35 had at least 2 cycles of therapy. Partial response was observed in 5 patients (11%, estimated 95% confidence interval 2–20%), with a median duration of 13 months. All responding patients had stabilization or improvement in performance status. Fourteen patients had stable disease of 4 or more months. The median WBC nadir was 3.8 × 103/μl (range 1.6–9.3) and the median absolute neutrophil (ANC) nadir was 2.0 × 103/μl (range 0.4–7.2). Thrombocytopenia - 100.0 × 103/μl was observed in 15 patients; the median platelet nadir was 123.0 (range 30.0–245.0). All patients experienced a mild to moderate flu-like syndrome. In addition, one patient had a mild hemolytic-uremic syndrome which appeared related to gemcitabine therapy. Gemcitabine demonstrated marginal activity in this resistant neoplasm, without excessive toxicity. Further evaluation, including the use of more intense dosing and/or combination therapy, is warranted.
Similar content being viewed by others
References
Boring CC, Squires TS, Long T: Cancer Statistics. CA 41:19–36, 1991
Brennan MF, Kinsella T, Friedman M: Cancer of the Pancreas. In: Debita VT Jr, Hellman S, Rosenberg SA (eds). Cancer: Principles and Practice of Oncology. 3rd edition. Philadelphia: JB Lippincott Company, 800–835, 1989
Hertel LW, Boder GB, Kroin JS, Rinzel SM, Poore GA, Todd GC, Grindey GB: Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer Res 50:4417–4422, 1990
Heinemann V, Hertel L, Grindey GB, Plunkett W: Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluorodeoxycytidine and 1-ß-D-arabinofuranosylcytosine. Cancer Res 48:4024–4031, 1988
Ghandi V, Plunkett W: Modulatory acitivity of 2′,2′-difluorodeoxycytidine on the phosphorylation and cytotoxicity of arabinosyl nucleosides. Cancer Res 50:3675–3680, 1990
Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W: Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res 51:6110–6117, 1991
Data on File. Eli Lilly Company, Indianapolis, IN
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, Plunkett W: A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 9:491–498, 1991
Miller AB, Hoogstraten B, Staquet M: Reporting the results of cancer treatment. Cancer 47:207–214. 1981
Fleming TR: One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151, 1982
Cantrell JE Jr, Philips TM, Schein PS: Carcinoma-associated hemolytic-uremic syndrome: a complication of mitomycin C chemotherapy. J Clin Oncol 5:723–734, 1985
Kyriazis AP, Kyriazis AA, Yagoda AA: Enhanced therapeutic effect of cis-diamminodichloroplatinum against nude mouse grown human pancreatic adenocarcinoma when combined with I-B-D arabionfuranosylcytosine and caffeine. Cancer Res 45:6083–6087, 1985
Heinemann V, Xu Y-Z, Chubb S, Sen A, Hertel LW, Grindey GB, Plunkett W. Cellular elimination of 2′,2′-difluordeoxycytidine 5′-triphosphate: a mechanism of self-potentiation. Cancer Res 52:533–539, 1992
Grunewald R, Kantarjian H, Keating MJ, Abbruzzese J, Tarassoff P, Plunkett W. Pharmacologically directed design of the dose rate and schedule of 2′-2′-difluordexoycytidine (gemcitabine) administration in leukemia. Cancer Res 50:6823–6826, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casper, E.S., Green, M.R., Kelsen, D.P. et al. Phase II trial of gemcitabine (2,2′-difiuorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs 12, 29–34 (1994). https://doi.org/10.1007/BF00873232
Issue Date:
DOI: https://doi.org/10.1007/BF00873232