Abstract
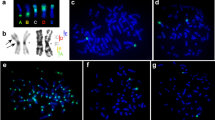
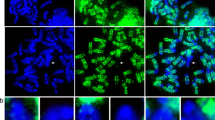
Mouse chromosomes possessing multiple Robertsonian rearrangements (Rb chromosomes) have been examined using fluorescencein situ hybridization with the telomeric consensus sequence (TTAGGG)n. No hybridization signals were detected at the primary constriction of Rb chromosomes. This observation leads us to conclude that the formation of Rb chromosomes in the mouse is invariably associated with the loss of telomeric regions. More significantly, a further alteration in regions flanking the primary constrictions was observed after hybridizing with a minor satellite DNA probe to Rb chromosomes. It seems likely that the breakpoints required for a Robertsonian process do not include telomeric sites exclusively but extend to the adjacent pericentromeric regions of the original acrocentric chromosomes. In contrast to previous reports, these observations demonstrate the elimination of substantial amounts of chromosomal DNA during the formation of mouse Rb chromosomes.
Similar content being viewed by others
References
Ashley T, Ward DC (1993) A ‘hot spot’ of recombination coincides with an interstitial telomeric sequence in the Armenian hamster.Cytogenet Cell Genet 62: 169–171.
Baker RJ, Bickham JW (1986) Speciation by monobrachial centric fusions.Proc Natl Acad Sci USA 83: 8245–8248.
Belkhir K, Britton-Davidian J, Konig B (1991) De nouvelles populations robertsoniennes de souris (Mus musculus domesticus) au nord des Alpes.Genome 34: 658–660.
Blin N, Stafford DW (1976) A general method for isolation of high molecular weight DNA from eukaryotes.Nucleic Acids Res 3: 2303–2308.
Broccoli D, Miller OJ, Miller D (1990) Relationship of mouse minor satellite DNA to centromere activity.Cytogenet Cell Genet 54: 182–186.
Capanna E (1982) Robertsonian numerical variation in animal speciation:Mus musculus, an emblematic model. In: Barigozzi C, ed.Mechanisms of Speciation. New York: Alan R Liss, pp 155–177.
Capanna E, Gropp A, Winking H, Noack G, Civitelli MV (1976) Robertsonian metacentrics in the mouse.Chromosoma 58: 341–353.
Carpenter ATC (1987) Gene conversion, recombination nodules, and the initiation of meiotic synapsis.BioEssays 6: 232–236.
Comings DE, Avelino E (1972) DNA loss during Robertsonian fusion in studies of the Tobacco mouse.Nature New Biol 237: 199.
Comings DE, Okada TA (1970) Whole-mount electron microscopy of the centromeric region of metacentric and telocentric mammalian chromosomes.Cytogenetics 9: 436–449.
Eicher EM, Lee BK, Washburn LL, Hale DW, King TR (1992) Telomere-related markers for the pseudoautosomal region of the mouse genome.Proc Natl Acad Sci USA 89: 2160–2164.
Elder FFB, Hsu TC (1988) Tandem fusion in the evolution of mammalian chromosomes. In: Daniel A, ed.The Cytogenetics of Mammalian Autosomal Rearrangements. New York: Alan R Liss, pp 481–506.
Elliott RW, Yen C-H (1991) DNA variants with telomere probe enable genetic mapping of ends of mouse chromosomes.Mammalian Genome 1: 118–122.
Garagna S, Redi CA, Capanna Eet al. (1993) Genome distribution, chromosomal allocation and organization of the major and minor satellite DNAs in 11 species and subspecies of the genusMus.Cytogenet Cell Genet 64: 247–255.
Gropp A, Tettenborn U, Lehmann E (1970) Chromosomenvariation vom Robertsonschen Typus bei der TabakmausM. poschiavinus und ihren hybriden mit der laboratoriumsmaus.Cytogenetics 9: 9–23.
Gropp A, Winking H, Zech L, Müller HJ (1972) Robertsonian chromosomal variation and identification of metacentric chromosomes in feral mice.Chromosoma 39: 265–288.
Guttenbach M, Schmid M (1990) Determination of Y chromosome aneuploidy in human sperm nuclei by nonradioactivein situ hybridization.Am J Hum Genet 46: 553–558.
Hastie ND, Allshire RC (1989) Human telomeres: fusion and interstitial sites.Trends Genet 5: 326–331.
Holmquist G, Dancis BM (1980) A general model of karyotype evolution.Genetica 52/53: 151–163.
Hsu TC, Patton JL (1969) Bone marrow preparations for chromosome studies. In: Benrischke K, ed.Comparative Mammalian Cytogenetics. Berlin: Springer, pp 454–460.
Hsu TC, Pathak S, Chen TR (1975) The possibility of latent centromeres and a proposed nomenclature system for total chromosome and whole arm translocations.Cytogenet Cell Genet 15: 41–49.
Ijdo JW, Baldini A, Ward DC, Reeders ST, Wells RA (1991) Origin of human chromosome 2: An ancestral telomere-telomere fusion.Proc Natl Acad Sci USA 88: 9051–9055.
John B, Freeman M (1975) Causes and consequences of Robertsonian exchange.Chromosoma 52: 123–136.
Kipling D, Ackford HE, Taylor BA, Cooke HJ (1991) Mouse minor satellite DNA genetically maps to the centromere and is physically linked to the proximal telomere.Genomics 11: 235–241.
Matthew R (1965) Cytogenetic mechanism and speciations of mammals.In vitro 1: 1–11.
Meyne J, Ratliff RL, Moyzis RK (1989) Conservation of the human telomere sequence (TTAGGG) n among vertebrates.Proc Natl Acad Sci USA 89: 7049–7053.
Meyne J, Baker RJ, Hobart HHet al. (1990) Distribution of non-telomeric sites of the (TTAGGG) n telomeric sequence in vertebrate chromosomes.Chromosoma 99: 3–10.
Miller OJ, Miller DA, Tantravahi R, Dev VG (1978) Nucleolus organizer activity and the origin of Robertsonian translocation.Cytogenet Cell Genet 20: 40–50.
Moyzis RK, Buckingham JM, Cram LSet al. (1988) A highly conserved repetitive DNA sequence (TTAGGG) n present at the telomeres of human chromosomes.Proc Natl Acad Sci USA 85: 6622–6626.
Narayanswami S, Doggett NA, Clark LM, Hildebrand CE, Weier H-U, Hamkalo BA (1992) Cytological and molecular characterization of centromeres inMus domesticus andMus spretus.Mammalian Genome 2: 186–194.
Park VM, Gustashaw KM, Wathen TM (1992) The presence of interstitial telomeric sequences in constitutional chromosome abnormalities.Am J Hum Genet 50: 914–923.
Pluta AF, Zakian VA (1989) Recombination occurs during telomere formation in yeast.Nature 337: 429–433.
Redi CA, Garagna S, Mazzini S, Winking H (1986) Pericentromeric heterochromatin and A-T contents during Robertsonian fusion in the house mouse.Chromosoma 94: 31–35.
Redi CA, Garagna G, Zuccotti M (1990) Robertsonian chromosome formation and fixation: the genomic scenario.Biol J Linn Soc 41: 235–255.
Reimann N, Rogalla P, Kazmierczak Bet al. (1994) Evidence that metacentric and submetacentric chromosomes in canine tumors can result from telomeric fusions.Cytogenet Cell Genet 67: 81–85.
Robertson WRB (1916) Chromosome studies. I. Taxonomic relationships shown in the chromosomes of tettigidae and acrididae: V-shaped chromosomes and their significance in acrididae, locustidae, and gryllidae: chromosomes and variation.J Morphol 27: 179–331.
Rossi E, Floridia G, Casali Met al. (1993) Types, stability, and phenotype consequences of chromosome rearrangements leading to interstitial telomeric sequences.J Med Genet 30: 926–931.
Seabright M (1971) A rapid banding technique for human chromosomes.Lancet ii: 971–972.
Scherthan H (1990) Localization of the repetitive telomeric sequence (TTAGGG) n in two muntjac species and implications for their karyotypic evolution.Cytogenet Cell Genet 53: 115–117.
Schmid M, Feichtinger W, Nanda Iet al. (1994) An extraordinary low diploid chromosome number in the reptileGonatodes taniae (Sauria, Gekonidae) J Hered 85: 255–260.
Schubert I, Schriever-Schwemmer G, Werner T, Adler I-D (1992) Telomeric signals in Robertsonian fusion and fission chromosomes: implication for the origin of pseudoaneuploidy.Cytogenet Cell Genet 59: 6–9.
Starling JA, Maule J, Hastie ND, Allshire RC (1990) Extensive telomere repeat arrays in mouse are hypervariable.Nucleic Acids Res 18: 6881–6888.
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin.Exp Cell Res 75: 304–306.
Tucker PK, Lee BK, Lundrigan BL, Eicher EM (1992) Geographic origin of the Y chromosomes in ‘old’ inbred strains of mice.Mammalian Genome 3: 254–261.
White MJD (1973)Animal Cytology and Evolution. Cambridge: Cambridge University Press.
Winking H, Beatrica D, Bulfield G (1988) Robertsonian karyotype variation in the European house mouse,Mus musculus: survey of present knowledge and new observations.Z Säugetierkunde 53: 148–161.
Wong AKC, Rattner JB (1988) Sequence organization and cytological localization of the minor satellite of mouse.Nucleic Acids Res 16: 11645–11661.
Wong AKC, Biddle FG, Rattner JB (1990) The chromosomal distribution of the major and minor satellite is not conserved in the genusMus.Chromosoma 99: 190–195.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nanda, I., Schneider-Rasp, S., Winking, H. et al. Loss of telomeric sites in the chromosomes ofMus musculus domesticus (Rodentia: Muridae) during Robertsonian rearrangements. Chromosome Res 3, 399–409 (1995). https://doi.org/10.1007/BF00713889
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00713889