Summary
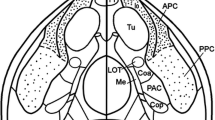
The spatial organization and laminar distribution of projections from the olfactory bulb and the anterior (PPCa) and posterior (PPCp) divisions of the prepiriform cortex to the entorhinal cortex were studied with anterograde (3H-leucine) and retrograde (WGA-HRP) tracing techniques. After 3H-leucine injections into the olfactory bulb transported labeling was seen over the lateral entorhinal area, except its most medial part, and over the rostral part of the medial entorhinal area. The labeling covers exclusively layer Ia. The lateral and medial entorhinal areas are also reached by fibers from the prepiriform cortex. The projection to the medial entorhinal area has not been described previously. Following injections of 3H-leucine into the PPCa transported labeling is present over the entire expanse of the entorhinal cortex and is located over layer Ib with the greatest density in its superficial part. Injections of 3H-leucine into the PPCp give rise to transported labeling over much of the entorhinal cortex. No labeling was found over the most medial parts of the medial subdivision (VMEA) of the lateral entorhinal area and the medial entorhinal area. Labeling occupies layer Ib, especially its middle part, and layers II and III. Both PPCa and PPCp appear to project most heavily to the dorsal (DLEA) and ventral (VLEA) subdivisions of the lateral entorhinal area. From the retrograde experiments it can be inferred that cells of layers II and III of the PPCa project predominantly to the DLEA, whereas those of the PPCp project predominantly to the VLEA. The MEA receives its heaviest projection from layer II of both PPCa and PPCp. In control experiments with 3H-leucine injections into the endopiriform nucleus it was found that this nucleus projects to the entire expanse of the entorhinal cortex. The fibers distribute to all layers with the exception of layer Ia.
Similar content being viewed by others
Abbreviations
- AI:
-
agranular insular cortex
- AL:
-
lateral nucleus of the amygdala
- BL:
-
basolateral nucleus of the amygdala
- BM:
-
basomedial nucleus of the amygdala
- C:
-
claustrum
- CoA:
-
cortical nucleus of the amygdala
- DLEA:
-
dorsal division of the lateral entorhinal cortex
- END:
-
endopiriform nucleus
- H:
-
hippocampus
- I:
-
granular insular cortex
- lot:
-
lateral olfactory tractus
- MCL:
-
mitral cell layer of the olfactory bulb
- MEA:
-
medial entorhinal area
- OB:
-
olfactory bulb
- PPCa:
-
anterior part of the prepiriform nucleus
- PPCp:
-
posterior part of the prepiriform nucleus
- VLEA:
-
ventral division of the lateral entorhinal cortex
- VMEA:
-
ventromedial division of the lateral entorhinal cortex
- 35:
-
area 35 of the perirhinal cortex
- 36:
-
area 36 of the perirhinal cortex
References
Beckstead R (1978) Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res 152: 249–264
Blackstad TW (1972) Commissural connections of the hippocampal region in the rat with special reference to their mode of termination. J Comp Neurol 105: 417–539
Boeijinga PH, Groen Th van, Lopes da Silva FH, Room P, Russchen FT (1982) Inputs from primary olfactory cortex to the entorhinal area: an electrophysiological and anatomical study. Neurosci Lett (Suppl) 10: S 82
Boeijinga PH, Groen Th van (1984) Inputs from olfactory bulb and olfactory cortex in cat: II Physiological studies. Exp Brain Res (submitted)
Broadwell RD (1975) Olfactory relations of the telencephalon and diencephalon in the rabbit. J Comp Neurol 163: 329–346
Dennis BJ, Kerr DIB (1968) An evoked potential study of centripetal and centrifugal connections of the olfactory bulb in the cat. Brain Res 11: 373–396
Haberly LB, Price JL (1978) Association and commissural fiber systems of the olfactory cortex of the rat. I: Systems originating in the piriform cortex and adjacent areas. J Comp Neurol 178: 711–740
Habets AMMC (1980) The projection of the prepiriform cortex to the hippocampus in the cat. Dissertation. University of Utrecht, the Netherlands
Habets AMMC, Lopes da Silva FH, Mollevanger WJ (1980) An olfactory input to the hippocampus of the cat: Field potential analysis. Brain Res 182: 47–64
Heimer L (1968) Synaptic distribution of centripetal and centrifugal nerve fibers in the olfactory system of the rat. An experimental anatomical study. J Anat 103: 413–432
Heimer L (1978) The olfactory cortex and the ventral striatum. In: Livingston, Hornykiewicz (eds): Limbic Mechanisms. New York, Plenum Press, pp 95–189
Hess DT, Schneider GE (1981) Advantage of polarization microscopy in HRP neurochemistry. J Histochem Cytochem 29: 1448
Illing RB, Wässle H (1979) Visualization of the HRP reaction product using the polarization microscope. Neurosci Lett 13: 7–11
Kerr DIB, Dennis J (1972) Collateral projections of the lateral olfactory tract to entorhinal cortical areas in the cat. Brain Res 36: 399–403
Kosel KC, Van Hoesen GW, West JR (1981) Olfactory bulb projections to the parahippocampal area of the rat. J Comp Neurol 198: 467–482
Krettek JE, Price JL (1977a) Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172: 687–722
Krettek JE, Price JL (1977b) Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in rat and cat. J Comp Neurol 172: 723–752
Krettek JE, Price JL (1978) A description of the amygdaloid complex in the rat and cat with observations on intraamygdaloid axonal connections. J Comp Neurol 178: 255–280
Luskin MB, Price JL (1983a) The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol 216: 264–291
Luskin MB, Price JL (1983b) The laminar distribution of intracortical fibers originating in the olfactory cortex of the rat. J Comp Neurol 216: 292–302
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Narkiewicz O (1964) Degeneration in the claustrum after regional neocortical ablations in the cat. J Comp Neurol 123: 335–356
Powell TPS, Cowan WM, Raisman G (1965) The central olfactory connections. J Anat 99: 791–813
Price JL (1973) An autoradiographic study of complementory laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol 150: 87–108
Price JL, Powell TPS (1971) Certain observations on the olfactory pathways. J Anat 110: 105–126
Reinoso-Suarez F (1961) Topographischer Hirnatlas der Katze für experimental-physiologische Untersuchungen. E Merck AG, Darmstadt
Scalia F (1966) Some olfactory pathways in the rabbit brain. J Comp Neurol 126: 285–310
Scalia F, Winans SS (1975) The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol 161: 31–56
Skeen LC, Hall WC (1977) Efferent projections of the main and the accessory olfactory bulb in the tree shrew (Tupaia glis). J Comp Neurol 72: 1–36
Snider RS, Niemer WT (1961) A stereotaxic atlas of the cat brain. The University of Chicago Press, Chicago
Turner BH, Gupta KC, Mishkin M (1978) The locus and cytoarchitecture of the projection areas of the olfactory bulb in Macaca mulatta. J Comp Neurol 177: 381–396
Van Hoesen GW, Pandya DN (1972) Cortical afferents in the entorhinal cortex of the Rhesus monkey. Science. 175: 1471–1473
White LE Jr (1965) Olfactory bulb projections of the rat. Anat Rec 152: 465–480
Wilson RC, Steward O (1978) Poly-synaptic activation of the dentate gyms of the hippocampal formation: An olfactory input via the lateral entorhinal cortex. Exp Brain Res 33: 523–534
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Room, P., Groenewegen, H.J. & Lohman, A.H.M. Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. Exp Brain Res 56, 488–496 (1984). https://doi.org/10.1007/BF00237989
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00237989