Abstract
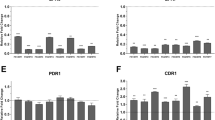
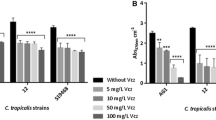
This study aimed to establish the influence of biofilm from clinical isolates of Candida albicans on fluconazole resistance, focusing on efflux pumps and azole-targeted enzymes. Twenty-three C. albicans clinical isolates were collected from two hospitals in Shanghai, China. Antifungal susceptibility tests were performed on biofilm and planktonic cells. A crystal violet assay was used to monitor biofilm growth. Real-time RT-PCR was performed to quantify the expression of the transporter-related genes MDR1, CDR1, and CDR2 as well as ERG11, a gene encoding an enzyme targeted by antifungal drugs. Fluconazole resistance was shown to increase in biofilm in a time-dependent manner. No significant differences were observed between different strains of C. albicans. Genes encoding efflux pumps were overexpressed in early stages of biofilm formation and could also be induced by fluconazole. While ERG11 was not upregulated in biofilm, it was overexpressed upon the addition of fluconazole to biofilm and planktonic cells. Gene expression also appeared to be related to the original genotype of the strain. The upregulation of genes encoding efflux pumps demonstrates their role in the development of fluconazole resistance during the early stages of C. albicans biofilm formation.




Similar content being viewed by others
References
Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M (2011) Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J Clin Microbiol 49:396–399
Ramage G, Martínez JP, López-Ribot JL (2006) Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6:979–986
Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA (2001) Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res 80:903–908
Hawser SP, Douglas LJ (1995) Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39:2128–2131
Nailis H, Kucharíková S, Řičicová M, Van Dijck P, Deforce D, Nelis H, Coenye T (2010) Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and-independent gene expression. BMC Microbiol 10:114
Mateus C, Crow SA, Ahearn DG (2004) Adherence of Candida albicans to silicone induces immediate enhanced tolerance to fluconazole. Antimicrob Agents Chemother 48:3358–3366
Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA (2003) Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun 71:4333–4340
Ramage G, VandeWalle K, Bachmann SP, Wickes BL, López-Ribot JL (2002) In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother 46:3634–3636
Al-Fattani MA, Douglas LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55:999–1008
Baillie GS, Douglas LJ (2000) Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother 46:397–403
Hawser SP, Baillie GS, Douglas LJ (1998) Production of extracellular matrix by Candida albicans biofilm. J Med Microbiol 47:253–256
Mitchell KF, Taff HT, Cuevas MA, Reinicke EL, Sanchez H, Andes DR (2013) Role of matrix β-1, 3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob Agents Chemother 57:1918–1920
Peeters E, Nelis HJ, Coenye T (2008) Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72:157–165
Calışkan S, Keçeli ÖS, Cınar S, Corakçı A, Calışkan E (2011) In vitro biofilm formation and relationship with antifungal resistance of Candida spp. isolated from vaginal and intrauterine device string samples of women with vaginal complaints. Mikrobiyol Bul 45:697–706
Douglas LJ (2002) Medical importance of biofilms in Candida infections. Rev Iberoam Micol 19:139–143
Douglas LJ (2013) Candida biofilms and their role in infection. Trends Microbiol 11:30–36
Uppuluri P, Srinivasan A, Ramasubramanian A, Lopez-Ribot JL (2011) Effects of fluconazole, amphotericin B, and caspofungin on Candida albicans biofilms under conditions of flow and on biofilm dispersion. Antimicrob Agents Chemother 55:3591–3593
Vavala E, Colone M, Passariello C, Celestino I, Toccacieli L, Stringaro A, Angiolella L (2013) Characterization of biofilms in drug-sensitive and drug-resistant strains of Candida albicans. J Chemother 25:87–95
Ben-Yaacov R, Knoller S, Caldwell GA, Becker JM, Koltin Y (1994) Candida albicans gene encoding resistance to benomyl and methotrexate is a multidrug resistance gene. Antimicrob Agents Chemother 38:648–652
Marger MD, Saier MH Jr (1993) A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci 18:13–20
Prasad R, De Wergifosse P, Goffeau A, Balzi E (1995) Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet 27:320–329
Coste AT, Karababa M, Ischer F, Bille J, Sanglard D (2004) TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell 3:1639–1652
Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL (2002) Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother 49:973–980
Perumal P, Mekala S, Chaffin WLJ (2007) Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother 51:2454–2463
White TC (1997) Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 41:1482–1487
Borecká-Melkusová S, Moran GP, Sullivan DJ, Kucharíková S, Chorvát D Jr, Bujdakova H (2009) The expression of genes involved in the ergosterol biosynthesis pathway in Candida albicans and Candida dubliniensis biofilms exposed to fluconazole. Mycoses 52:118–128
Nailis H, Vandenbosch D, Deforce D, Nelis HJ, Coenye T (2010) Transcriptional response to fluconazole and amphotericin B in Candida albicans biofilms. Res Microbiol 161:284–292
Acknowledgements
We thank our colleagues for their help with collecting the clinical isolates.
Funding
This work was supported by grants from the Program of Shanghai Municipal Health Bureau of China (2009239), the Program of Science and Technology Commission of Shanghai Municipality (114119b0500), and the Scientific Research Key Project of Shanghai Municipal Health Bureau (20124005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Carlos Taborda
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, C., Liu, J., Li, W. et al. Expression of fluconazole resistance-associated genes in biofilm from 23 clinical isolates of Candida albicans. Braz J Microbiol 50, 157–163 (2019). https://doi.org/10.1007/s42770-018-0009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-018-0009-2