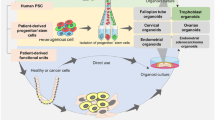
Abstract
The female reproductive system consists of the ovaries, the female gonads, and the reproductive tract organs of the fallopian tubes, uterus, cervix, and vagina. It functions to provide hormonal support and anatomical structure for the production of new offspring. A number of endogenous and exogenous factors can impact female reproductive health and fertility, including genetic vulnerability, medications, environmental exposures, age, nutrition, and diseases. To date, due to the ethical concerns of using human subjects in biomedical research, the majority of studies use in vivo animal models and 2D cell/tissue culture models to study female reproduction. However, the complexity and species difference of the female reproductive system in humans make it difficult to compare to those of animals. Moreover, the monolayered cells cultured on flat plastics or glass lose their 3D architecture as well as the physical and/or biochemical contacts with other cells in vivo. Further, all reproductive organs do not work alone but interconnect with each other and also with non-reproductive organs to support female reproductive, endocrine, and systemic health. These facts suggest that there is an urgent and unmet need to develop representative, effective, and efficient in vitro models for studying human female reproduction. The prodigious advancements of bioengineering (e.g., biomaterials, 3D printing, and organ-on-a-chip) allow us to study female reproduction in an entirely new way. Here, we review recent advances that use bioengineering methods to study female reproduction, including the bioengineering models of the ovary, fallopian tube, uterus, embryo implantation, placenta, and reproductive disease.






Similar content being viewed by others
References
Buyuk E, Nejat E, Neal-Perry G (2010) Determinants of female reproductive senescence: differential roles for the ovary and the neuroendocrine axis. Semin Reprod Med 28(5):370–379. https://doi.org/10.1055/s-0030-1262896
Prevention CfDCa (2019) Key statistics from the national survey of family growth: I listing. https://www.cdc.gov/nchs/nsfg/key_statistics/i_2015-2017.htm#infertility. Accessed 2020
Prevention CfDCa (2018) Common reproductive health concerns for women. https://www.cdc.gov/reproductivehealth/womensrh/healthconcerns.html
Organization WH (2020) Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed 2020
Tao JJ, Visvanathan K, Wolff AC (2015) Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast 24(Suppl 2):S149–153. https://doi.org/10.1016/j.breast.2015.07.035
Martin NE, D’Amico AV (2014) Progress and controversies: radiation therapy for prostate cancer. CA Cancer J Clin 64(6):389–407. https://doi.org/10.3322/caac.21250
Jeruss JS, Woodruff TK (2009) Preservation of fertility in patients with cancer. N Engl J Med 360(9):902–911. https://doi.org/10.1056/NEJMra0801454
Andersen CL, Liu M, Wang Z, Ye X, Xiao S (2018) Chemotherapeutic agent doxorubicin alters uterine gene expression in response to estrogen in ovariectomized CD-1 adult mice. Biol Reprod. https://doi.org/10.1093/biolre/ioy259
Havelock JC, Rainey WE, Carr BR (2004) Ovarian granulosa cell lines. Mol Cell Endocrinol 228(1–2):67–78. https://doi.org/10.1016/j.mce.2004.04.018
Pocar P, Augustin R, Gandolfi F, Fischer B (2003) Toxic effects of in vitro exposure to p-tert-octylphenol on bovine oocyte maturation and developmental competence. Biol Reprod 69(2):462–468. https://doi.org/10.1095/biolreprod.102.010355
Pocar P, Perazzoli F, Luciano AM, Gandolfi F (2001) In vitro reproductive toxicity of polychlorinated biphenyls: effects on oocyte maturation and developmental competence in cattle. Mol Reprod Dev 58(4):411–416. https://doi.org/10.1002/1098-2795(20010401)58:4%3c411:AID-MRD8%3e3.0.CO;2-R
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ (2002) Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296(5576):2178–2180. https://doi.org/10.1126/science.1071965
Chen J, Torcia S, Xie F, Lin CJ, Cakmak H, Franciosi F, Horner K, Onodera C, Song JS, Cedars MI, Ramalho-Santos M, Conti M (2013) Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol 15(12):1415–1423. https://doi.org/10.1038/ncb2873
Su YQ, Sugiura K, Eppig JJ (2009) Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 27(1):32–42. https://doi.org/10.1055/s-0028-1108008
Sugiura K, Pendola FL, Eppig JJ (2005) Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 279(1):20–30. https://doi.org/10.1016/j.ydbio.2004.11.027
Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG (2006) Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci 119(Pt 18):3811–3821. https://doi.org/10.1242/jcs.03105
Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK (2005) Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod 73(2):351–357. https://doi.org/10.1095/biolreprod.105.041798
Biggers JD, Whittingham DG, Donahue RP (1967) The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA 58(2):560–567
Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ (2008) Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135(1):111–121. https://doi.org/10.1242/dev.009068
Buccione R, Schroeder AC, Eppig JJ (1990) Interactions between somatic-cells and germ-cells throughout mammalian oogenesis. Biol Reprod 43(4):543–547. https://doi.org/10.1095/Biolreprod43.4.543
Gui UM, Joyce LA (2005) RNA interference evidence that growth differentiation factor-9 mediates clocyte regulation of cumulus expansion in mice. Biol Reprod 72(1):195–199. https://doi.org/10.1095/Biolreprod.104.033357
Oatley J, Hunt PA (2012) Of mice and (wo)men: purified oogonial stem cells from mouse and human ovaries. Biol Reprod 86(6):196. https://doi.org/10.1095/biolreprod.112.100297
Horan CJ, Williams SA (2017) Oocyte stem cells: fact or fantasy? Reproduction 154(1):R23–R35. https://doi.org/10.1530/REP-17-0008
Goswami D, Conway GS (2005) Premature ovarian failure. Hum Reprod Update 11(4):391–410. https://doi.org/10.1093/humupd/dmi012
Tibbitt MW, Anseth KS (2009) Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103(4):655–663. https://doi.org/10.1002/bit.22361
Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacik I, Shapiro AM, Pedraz JL (2003) Cell encapsulation: promise and progress. Nat Med 9(1):104–107. https://doi.org/10.1038/nm0103-104
Pangas SA, Saudye H, Shea LD, Woodruff TK (2003) Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng 9(5):1013–1021. https://doi.org/10.1089/107632703322495655
Xu M, Kreeger PK, Shea LD, Woodruff TK (2006) Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 12(10):2739–2746. https://doi.org/10.1089/ten.2006.12.2739
Kreeger PK, Fernandes NN, Woodruff TK, Shea LD (2005) Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod 73(5):942–950. https://doi.org/10.1095/biolreprod.105.042390
Hornick JE, Duncan FE, Shea LD, Woodruff TK (2013) Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 145(1):19–32. https://doi.org/10.1530/REP-12-0233
West ER, Xu M, Woodruff TK, Shea LD (2007) Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 28(30):4439–4448. https://doi.org/10.1016/j.biomaterials.2007.07.001
Xu M, West E, Shea LD, Woodruff TK (2006) Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 75(6):916–923. https://doi.org/10.1095/biolreprod.106.054833
Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK (2015) In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep 5:17323. https://doi.org/10.1038/srep17323
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32(12):3233–3243. https://doi.org/10.1016/j.biomaterials.2011.01.057
Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123(Pt 24):4195–4200. https://doi.org/10.1242/jcs.023820
Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK (2015) Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50:20–29. https://doi.org/10.1016/j.biomaterials.2015.01.051
Jakus AE, Laronda MM, Rashedi AS, Robinson CM, Lee C, Jordan SW, Orwig KE, Woodruff TK, Shah RN (2017) ”Tissue Papers” from organ-specific decellularized extracellular matrices. Adv Funct Mater. https://doi.org/10.1002/adfm.201700992
Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V (2016) First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol 214(1):94. https://doi.org/10.1016/j.ajog.2015.10.001
Oktay K, Taylan E, Kawahara T, Cillo GM (2019) Robot-assisted orthotopic and heterotopic ovarian tissue transplantation techniques: surgical advances since our first success in 2000. Fertil Steril 111(3):604–606. https://doi.org/10.1016/j.fertnstert.2018.11.042
Pors SE, Ramlose M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, Kristensen SG (2019) Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod 34(8):1523–1535. https://doi.org/10.1093/humrep/dez077
Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, Feinberg AW (2019) 3D bioprinting of collagen to rebuild components of the human heart. Science 365(6452):482–487. https://doi.org/10.1126/science.aav9051
Papaioannou TG, Manolesou D, Dimakakos E, Tsoucalas G, Vavuranakis M, Tousoulis D (2019) 3D bioprinting methods and techniques: applications on artificial blood vessel fabrication. Acta Cardiol Sin 35(3):284–289. https://doi.org/10.6515/ACS.201905_35(3).20181115A
Duan B, Hockaday LA, Kang KH, Butcher JT (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A 101(5):1255–1264. https://doi.org/10.1002/jbm.a.34420
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P (2014) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 20(6):473–484. https://doi.org/10.1089/ten.TEC.2013.0335
Markstedt K, Mantas A, Tournier I, Martinez Avila H, Hagg D, Gatenholm P (2015) 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromol 16(5):1489–1496. https://doi.org/10.1021/acs.biomac.5b00188
Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN (2017) A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 8:15261. https://doi.org/10.1038/ncomms15261
Raffel N, Dittrich R, Bauerle T, Seyler L, Fattahi A, Hoffmann I, Leal-Egana A, Beckmann MW, Boccaccini AR, Liverani L (2019) Novel approach for the assessment of ovarian follicles infiltration in polymeric electrospun patterned scaffolds. PLoS ONE 14(4):e0215985. https://doi.org/10.1371/journal.pone.0215985
Liverani L, Raffel N, Fattahi A, Preis A, Hoffmann I, Boccaccini AR, Beckmann MW, Dittrich R (2019) Electrospun patterned porous scaffolds for the support of ovarian follicles growth: a feasibility study. Sci Rep 9(1):1150. https://doi.org/10.1038/s41598-018-37640-1
Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR (2018) Bioinks for 3D bioprinting: an overview. Biomater Sci 6(5):915–946. https://doi.org/10.1039/c7bm00765e
Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32(8):760–772. https://doi.org/10.1038/nbt.2989
Ronaldson-Bouchard K, Vunjak-Novakovic G (2018) Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell 22(3):310–324. https://doi.org/10.1016/j.stem.2018.02.011
Sosa-Hernandez JE, Villalba-Rodriguez AM, Romero-Castillo KD, Aguilar-Aguila-Isaias MA, Garcia-Reyes IE, Hernandez-Antonio A, Ahmed I, Sharma A, Parra-Saldivar R, Iqbal HMN (2018) Organs-on-a-chip module: a review from the development and applications perspective. Micromachines. https://doi.org/10.3390/mi9100536
Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Nguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC, Getsios S, Burdette JE, Kim JJ, Borenstein JT, Woodruff TK (2017) A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 8:14584. https://doi.org/10.1038/ncomms14584
Nagashima JB, El Assal R, Songsasen N, Demirci U (2018) Evaluation of an ovary-on-a-chip in large mammalian models: species specificity and influence of follicle isolation status. J Tissue Eng Regen Med 12(4):e1926–e1935. https://doi.org/10.1002/term.2623
Eddy CA, Pauerstein CJ (1980) Anatomy and physiology of the fallopian tube. Clin Obstet Gynecol 23(4):1177–1193
Aviles M, Gutierrez-Adan A, Coy P (2010) Oviductal secretions: Will they be key factors for the future ARTs? Mol Hum Reprod 16(12):896–906. https://doi.org/10.1093/molehr/gaq056
Li S, Winuthayanon W (2017) Oviduct: roles in fertilization and early embryo development. J Endocrinol 232(1):R1–R26. https://doi.org/10.1530/JOE-16-0302
Eddie SL, Quartuccio SM, Zhu J, Shepherd JA, Kothari R, Kim JJ, Woodruff TK, Burdette JE (2015) Three-dimensional modeling of the human fallopian tube fimbriae. Gynecol Oncol 136(2):348–354. https://doi.org/10.1016/j.ygyno.2014.12.015
Zhu J, Xu Y, Rashedi AS, Pavone ME, Kim JJ, Woodruff TK, Burdette JE (2016) Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle. Mol Hum Reprod 22(11):756–767. https://doi.org/10.1093/molehr/gaw041
Ferraz M, Rho HS, Hemerich D, Henning HHW, van Tol HTA, Holker M, Besenfelder U, Mokry M, Vos P, Stout TAE, Le Gac S, Gadella BM (2018) An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat Commun 9(1):4934. https://doi.org/10.1038/s41467-018-07119-8
Psychoyos A (1973) Endocrine control of egg implantation. Handb Physiol 2:187–215
Norwitz ER, Schust DJ, Fisher SJ (2001) Implantation and the survival of early pregnancy. New England J Med 345(19):1400–1408. https://doi.org/10.1056/NEJMra000763
Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC (1988) Incidence of early loss of pregnancy. New England J Med 319(4):189–194. https://doi.org/10.1056/NEJM198807283190401
Cook CD, Hill AS, Guo M, Stockdale L, Papps JP, Isaacson KB, Lauffenburger DA, Griffith LG (2017) Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr Biol (Camb) 9(4):271–289. https://doi.org/10.1039/c6ib00245e
Olalekan SA, Burdette JE, Getsios S, Woodruff TK, Kim JJ (2017) Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol Reprod 96(5):971–981. https://doi.org/10.1093/biolre/iox039
Gnecco JS, Ding T, Smith C, Lu J, Bruner-Tran KL, Osteen KG (2019) Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium. Hum Reprod 34(4):702–714. https://doi.org/10.1093/humrep/dez003
Gnecco JS, Pensabene V, Li DJ, Ding T, Hui EE, Bruner-Tran KL, Osteen KG (2017) Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann Biomed Eng 45(7):1758–1769. https://doi.org/10.1007/s10439-017-1797-5
Pera MF (2017) Human embryo research and the 14-day rule. Development 144(11):1923–1925. https://doi.org/10.1242/dev.151191
Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL, Fu J (2019) Controlled modelling of human epiblast and amnion development using stem cells. Nature 573(7774):421–425. https://doi.org/10.1038/s41586-019-1535-2
Gracia C, Woodruff TK (2012) Oncofertility medical practice: clinical issues and implementation. Springer, New York
Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD, Mesas-Burgos C, Olive A, Caskey RC, Weiland TR, Han J, Schupper AJ, Connelly JT, Dysart KC, Rychik J, Hedrick HL, Peranteau WH, Flake AW (2017) An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun 8:15112. https://doi.org/10.1038/ncomms15112
Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G (2016) Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol 215(1 Suppl):S1–S46. https://doi.org/10.1016/j.ajog.2016.03.001
Mathiesen L, Mose T, Morck TJ, Nielsen JK, Nielsen LK, Maroun LL, Dziegiel MH, Larsen LG, Knudsen LE (2010) Quality assessment of a placental perfusion protocol. Reprod Toxicol 30(1):138–146. https://doi.org/10.1016/j.reprotox.2010.01.006
Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL (2006) In vitro models for studying trophoblast transcellular transport. Methods Mol Med 122:225–239. https://doi.org/10.1385/1-59259-989-3:225
Huang X, Luthi M, Ontsouka EC, Kallol S, Baumann MU, Surbek DV, Albrecht C (2016) Establishment of a confluent monolayer model with human primary trophoblast cells: novel insights into placental glucose transport. Mol Hum Reprod 22(6):442–456. https://doi.org/10.1093/molehr/gaw018
Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, Huh D (2016) Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 29(7):1046–1054. https://doi.org/10.3109/14767058.2015.1038518
Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, Huh D (2016) A microphysiological model of the human placental barrier. Lab Chip 16(16):3065–3073. https://doi.org/10.1039/c6lc00259e
Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, Aleksunes LM, Huh D (2018) Placental drug transport-on-a-chip: a microengineered in vitro model of transporter-mediated drug efflux in the human placental barrier. Adv Healthc Mater. https://doi.org/10.1002/adhm.201700786
Dabaghi M, Fusch G, Saraei N, Rochow N, Brash JL, Fusch C, Ravi Selvaganapathy P (2018) An artificial placenta type microfluidic blood oxygenator with double-sided gas transfer microchannels and its integration as a neonatal lung assist device. Biomicrofluidics 12(4):044101. https://doi.org/10.1063/1.5034791
Chen Z, Dai Y, Dong Z, Li M, Mu X, Zhang R, Wang Z, Zhang W, Lang J, Leng J, Jiang X (2012) Co-cultured endometrial stromal cells and peritoneal mesothelial cells for an in vitro model of endometriosis. Integr Biol (Camb) 4(9):1090–1095. https://doi.org/10.1039/c2ib00172a
Chen CH, Miller MA, Sarkar A, Beste MT, Isaacson KB, Lauffenburger DA, Griffith LG, Han J (2013) Multiplexed protease activity assay for low-volume clinical samples using droplet-based microfluidics and its application to endometriosis. J Am Chem Soc 135(5):1645–1648. https://doi.org/10.1021/ja307866z
Karolina Zuk A, Wen X, Dilworth S, Li D, Ghali L (2017) Modeling and validating three dimensional human normal cervix and cervical cancer tissues in vitro. J Biomed Res 31(3):240–247. https://doi.org/10.7555/JBR.31.20160150
Li SS, Ip CK, Tang MY, Sy SK, Yung S, Chan TM, Yang M, Shum HC, Wong AS (2017) Modeling ovarian cancer multicellular spheroid behavior in a dynamic 3D Peritoneal microdevice. J Vis Exp. https://doi.org/10.3791/55337
Kuznetsov L, Dworzynski K, Davies M, Overton C, Guideline C (2017) Diagnosis and management of endometriosis: summary of NICE guidance. BMJ 358:j3935. https://doi.org/10.1136/bmj.j3935
Watters KM, Bajwa P, Kenny HA (2018) Organotypic 3D models of the ovarian cancer tumor microenvironment. Cancers. https://doi.org/10.3390/cancers10080265
de Ferraz MAMM, Nagashima JB, Venzac B, Le Gac S, Songsasen N (2020) Author Correction: a dog oviduct-on-a-chip model of serous tubal intraepithelial carcinoma. Sci Rep 10(1):4733. https://doi.org/10.1038/s41598-020-61782-w
Dorayappan KDP, Gardner ML, Hisey CL, Zingarelli RA, Smith BQ, Lightfoot MDS, Gogna R, Flannery MM, Hays J, Hansford DJ, Freitas MA, Yu L, Cohn DE, Selvendiran K (2019) A Microfluidic chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian cancer. Cancer Res 79(13):3503–3513. https://doi.org/10.1158/0008-5472.CAN-18-3538
Fleszar AJ, Walker A, Kreeger PK, Notbohm J (2019) Substrate curvature induces fallopian tube epithelial cell invasion via cell-cell tension in a model of ovarian cortical inclusion cysts. Integr Biol (Camb) 11(8):342–352. https://doi.org/10.1093/intbio/zyz028
Fleszar AJ, Walker A, Porubsky V, Flanigan W, James D, Campagnola PJ, Weisman PS, Kreeger PK (2018) The extracellular matrix of ovarian cortical inclusion cysts modulates invasion of fallopian tube epithelial cells. APL Bioeng. https://doi.org/10.1063/1.5022595
Hannon PR, Flaws JA (2015) The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 6:8. https://doi.org/10.3389/fendo.2015.00008
Cha J, Sun X, Dey SK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18(12):1754–1767. https://doi.org/10.1038/nm.3012
Acknowledgements
This work is supported by the National Institutes of Health (NIH K01ES030014 and P01ES028942) and National Science Foundation (NSF 183291) to S. Xiao. We thank Jingshan Xu for contributing to the Figures and Supplemental Table 1. As the limited space, we apologize for not being able to include all previously published studies that contributed to the understanding of the bioengineering of female reproduction.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zubizarreta, M.E., Xiao, S. Bioengineering models of female reproduction. Bio-des. Manuf. 3, 237–251 (2020). https://doi.org/10.1007/s42242-020-00082-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-020-00082-8