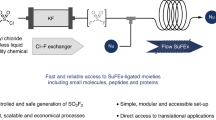
Abstract
Sulfonyl fluorides are valuable synthetic motifs which are currently of high interest due to the popularity of the sulfur (VI) fluoride exchange (SuFEx) click chemistry concept. Herein, we describe a flow chemistry approach to enable their synthesis through an electrochemical oxidative coupling of thiols and potassium fluoride. The reaction can be carried out at room temperature and atmospheric pressure and the yield of the targeted sulfonyl fluoride, by virtue of the short inter-electrode distance between a graphite anode and a stainless-steel cathode, reached up to 92% in only 5 min residence time compared to 6 to 36 h in batch. A diverse set of thiols (7 examples) was subsequently converted in flow. Finally, a fully telescoped process was developed which combines the electrochemical sulfonyl fluoride synthesis with a follow-up SuFEx reaction.
Similar content being viewed by others
Introduction
Click chemistry is a popular synthetic concept which enables the quick and reliable stitching of two molecular building blocks in high yield and selectivity. The concept has been coined by K.B. Sharpless [1] and has been widely employed in drug discovery [2], chemical biology, and material science as it is amenable to high-throughput experimentation. In general, click chemistry is a collection of synthetic methods that are high yielding, fast, easy to perform and produce little to no byproducts. One of the most popular click reactions is the Cu(I)-catalyzed azide-alkyne cycloaddition which yields triazoles [3]. More recently, a new click reaction was developed by Sharpless and coworkers, i.e. sulfur (VI) fluoride exchange (SuFEx) which employs sulfonyl fluorides as stable and robust reagents [4].
Key to the success of the click chemistry concept is the access to a broad array of structurally diverse click reagents in large quantities [5]. It is general belief that flow chemistry can be particularly helpful in realizing this objective. As an example, the synthesis of azides has been reported by many research groups and has been successfully coupled with the follow-up Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) [6,7,8,9,10]. The combination of these two steps leads to a significant time reduction and keeps the total inventory of hazardous azides low, thus effectively reducing the safety risks associated with these reagents [11]. Moreover, the use of superheated reaction conditions in combination with copper-based capillaries allows to further reduce the reaction time effectively [12]. Removal of the homogeneous Cu(I)-catalyst can also be achieved in flow leading to almost pure triazole compounds, which meet the stringent product purity requirements needed in the pharmaceutical industry [13, 14].
While the CuAAC has served as a benchmark reaction for flow chemistry in the past two decades, SuFEx has received much less scrutiny. Recently, our group developed an electrochemical approach to access the key sulfonyl fluoride starting materials [15]. The method involves an anodic oxidation process and uses widely available thiols or disulfides and KF as a cheap, safe and widely available fluorine source. Biphasic reaction conditions (acetonitrile/1 M HCl) are required and the reaction was carried out in batch. However, preliminary studies showed that the use of flow chemistry was of great benefit [16,17,18,19,20]. The reaction time could be reduced to the minute range in flow and no mass transfer limitations were observed. The reduced reaction time can be attributed to (i) the increased electrode surface-to-volume ratio, (ii) a high interfacial area between the organic and aqueous phase, and (iii) intensified mass transport due to multiphase fluid patterns.
In this manuscript, we provide a full investigation of all relevant process parameters in the flow-enabled electrochemical oxidative coupling of thiols and fluoride yielding sulfonyl fluorides. Moreover, we have for the first time coupled the sulfonyl fluoride synthesis with a subsequent SuFEx click reaction in flow, which represents a particularly useful strategy to handle the most volatile sulfonyl fluoride reagents.
Results and discussion
Initial experiments were carried out with thiophenol as the benchmark substrate and KF as the fluoride source (Scheme 1). It should be noted that all experiments described in this paper are carried out in a home-built electrochemical flow reactor (Fig. 1) [21]. At the cathode, hydrogen is generated as a benign and high value byproduct. In such a scenario, electrodes with a low hydrogen overpotential are typically preferred, e.g. platinum, copper or stainless steel [22]. From our previous experiments in batch, we found that a graphite anode and a stainless steel cathode worked optimal and this proved to be also the case in our flow experiments (Table 1, Entry 1). Other electrode materials as cathode, such as copper, graphite or nickel, did not lead to any improvement (Table 1, Entries 2–4).
Next, we investigated the influence of the solvent system (Table 2). As the organic phase, acetonitrile was selected as the optimal solvent. Lower yields for the target product were obtained in other common organic solvents, such as THF or methanol (Table 2, Entries 6–7). The presence of acid provided in general higher yields compared to non-acidic reaction mixtures (Table 2, Entry 1). However, the presence of sulfuric acid proved to be detrimental for the reaction as no product formation was observed (Table 2, Entry 2).
Next, we investigated the influence of the fluoride source on the reaction outcome (Table 3). As shown in our previous work [15], the reaction worked well with alkali fluorides but also with Selectfluor. The use of Selectfluor was not further considered due to low atom efficiency and its higher price compared to alkali fluorides. The reaction worked best with 5 equivalents of KF (Table 3, Entry 4). While only one equivalent is needed for the reaction, the remaining 4 equivalents served as a cheap supporting electrolyte. Increasing the amount of KF did not lead to further improvements (Table 3, Entries 6–7).
The electrochemical oxidative coupling of thiols and potassium fluoride requires the addition of pyridine to obtain high yields. It is possible that pyridine functions either as an electron mediator [23] or a as phase transfer catalyst. From our mechanistic investigations [15], we believe that at least pyridine functions partly as a phase transfer catalyst (Table 4). Screening the concentration of pyridine, we found that the best results were obtained with 0.6–0.7 M pyridine (Table 4, Entries 6 and 9). Other phase transfer catalysts, such as tetrabutyl ammonium bromide and chloride were less effective (Table 4, Entries 7–8).
Electrochemical transformations can be carried out either potentiostatic (constant potential) or galvanostatic (constant current). Under both scenarios, excellent yields could be obtained in flow as can been seen from Fig. 2. However, the highest yield and the most stable operation was observed under potentiostatic reaction conditions with GC yields up to 92%. Galvanostatic reactions provide a constant current and thus the reaction rate is constant until complete conversion is obtained. In contrast, potentiostatic operation keeps the cell potential constant and is of high interest to obtain high and tunable reaction selectivity [24]. While galvanostatic operation is preferred in batch, potentiostatic reaction conditions in flow are in our experience equally fast [25, 26]. We believe this has to do with the fact that the conversion increases along the channel length and thus a constant supply of electrons is maintained the entire time. Indeed, during a four-hour stability test, we saw that the current remained constant between 400 and 500 mA (Fig. 3). This is in contrast with batch potentiostatic experiments where the supply of electrons decreases when the conversion increases (less product needs to be converted, thus higher potential and lower current). This leads to slower reaction rates towards the end of the reaction and thus full conversion is harder to reach. In batch, this is often solved by adding large amounts of supporting electrolyte.
Stability test (4 h operation time) for the reaction (conditions are those from Scheme 1) with a 5 min residence time. GC-yield using GC-FID with internal standard (biphenyl)
While the reaction required 24–36 h in batch to reach full conversion [15], the reaction can be completed in only 5 min in flow (Figs. 4 and 5). Such intensified reaction conditions can be attributed to the short diffusion distances to the electrode surface, the intensified mass transport due to multiphase flow patterns and the increased interfacial area.
Scale-up test (2 mmol to 10 mmol scale) for the reaction with 5 min residence time. Conditions, see Scheme 1. Yields reported are those of isolated products
Next, we investigated the scalability of our flow protocol. Since electrochemical transformations are surface reactions, scale-up in batch can be regarded as very challenging. Typically, the electrode size is increased and larger amounts of supporting electrolyte are required to cope with the increase in Ohmic drop. Therefore, nearly all industrial electrochemical processes are carried out as flow processes in narrow-gap cells (inter-electrode gap = 0.5–10 mm) which are numbered-up depending on the targeted throughput [16]. As can be seen from Fig. 5, the GC and isolated yields remained constant from 2 mmol to 10 mmol scale, showing proof that the reaction is scalable but also provides stable output for longer periods of time.
With the optimal reaction conditions in hand, we commenced evaluating the flow protocol for the conversion of a diverse set of thiols (Scheme 2). In most scenarios, the yields were comparable with the batch protocol. It should be noted that no individual optimization was carried out, this explains that in some cases a slightly lower yield was obtained compared to individually optimized batch examples. Nevertheless, the results shown in Scheme 2 show clearly that the batch protocols can be seamlessly translated to flow.
Flow synthesis of sulfonyl fluorides and comparison with the batch protocol. Reaction conditions in flow: 0.1 M thiol, 0.6 M pyridine, 0.5 M KF, 3.30 V, 1 M HCl/ CH3CN (1:1 v/v), total flow rate 150 μL/min, residence time 5 min; Reaction conditions in batch: 2 mmol thiol, 10 mmol KF, 8 mmol pyridine, 10 mL CH3CN, 10 mL 1 M HCl, 3.20 V. Yields reported are those of isolated products
Some of the corresponding sulfonyl fluorides presented in Scheme 2 are volatile and therefore difficult to isolate on small scale. Immediate conversion of these compounds in a follow-up SuFEx-type transformation is therefore recommended. In flow, this can be easily achieved by combining the individual steps in a single, uninterrupted flow protocol [27]. Hereto, the sulfonyl fluoride product (92% GC yield, 82% isolated yield) exiting the electrochemical reactor is first quenched with a saturated NaHCO3 solution as the SuFEx reaction requires to be carried out at neutral pH (Scheme 3). Sampling points were added in our design to verify the yield of the sulfonyl fluoride (Scheme 3, Sampling point 1) and the pH (Scheme 3, Sampling point 2). Next, the neutralized reaction stream was merged with a reagent stream containing phenol and a stream containing additional Cs2CO3. Within 30 s, we observed a clean conversion to the corresponding phenyl sulfonate derivative (73% isolated yield) using this telescoped flow process. Moreover, this example highlights the clear advantage of combining the reagent synthesis and the subsequent SuFEx click reaction in a telescoped fashion in terms of isolation, time and labor reduction. Further, we believe this process should be amenable to applications in high throughput experimentation [28, 29].
In conclusion, a continuous-flow protocol for the electrochemical oxidative coupling of thiols and KF to prepare sulfonyl fluorides was developed. The flow protocol leads to significant shorter reaction times (5 min in flow vs. 6–36 h in batch) and proves to be scalable (up to 10 mmol). Moreover, the flow sulfonyl fluoride synthesis can be readily telescoped into a SuFEx follow up reaction, without the need for intermediate purification. Ultimately, we believe that this flow protocol will be useful for those in academia and industry, interested in SuFEx click chemistry.
References
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chemie Int Ed 40:2004–2021
Thirumurugan P, Matosiuk D, Jozwiak K (2013) Click chemistry for drug development and diverse chemical–biology applications. Chem Rev 113:4905–4979
Liang L, Astruc D (2011) The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord Chem Rev 255:2933–2945
Dong J, Krasnova L, Finn MG et al (2014) Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew Chemie Int Ed 53:9430–9448
Meng G, Guo T, Ma T et al (2019) Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 574:86–89
Hatit MZC, Reichenbach LF, Tobin JM et al (2018) A flow platform for degradation-free CuAAC bioconjugation. Nat Commun 9:4021
Fuchs M, Goessler W, Pilger C et al (2010) Mechanistic insights into copper(I)-catalyzed Azide-alkyne Cycloadditions using continuous flow conditions. Adv Synth Catal 352:323–328
Bogdan AR, James K (2010) Efficient access to new chemical space through flow—construction of Druglike macrocycles through copper-surface-catalyzed Azide–alkyne Cycloaddition reactions. Chem – A Eur J 16:14506–14512
Borukhova S, Noël T, Metten B et al (2016) From alcohol to 1,2,3-triazole via a multi-step continuous-flow synthesis of a rufinamide precursor. Green Chem 18:4947–4953
Borukhova S, Noël T, Metten B et al (2013) Solvent- and catalyst-free Huisgen Cycloaddition to Rufinamide in flow with a greener, less expensive Dipolarophile. ChemSusChem 6:2220–2225
Kockmann N, Thenée P, Fleischer-Trebes C et al (2017) Safety assessment in development and operation of modular continuous-flow processes. React Chem Eng 2:258–280
Bogdan AR, Sach NW (2009) The use of copper flow reactor Technology for the Continuous Synthesis of 1,4-Disubstituted 1,2,3-Triazoles. Adv Synth Catal 351:849–854
Varas AC, Noël T, Wang Q et al (2012) Copper(I)-catalyzed Azide–alkyne Cycloadditions in microflow: catalyst activity, high-T operation, and an integrated continuous copper scavenging unit. ChemSusChem 5:1703–1707
Vural Gürsel I, Aldiansyah F, Wang Q et al (2015) Continuous metal scavenging and coupling to one-pot copper-catalyzed azide-alkyne cycloaddition click reaction in flow. Chem Eng J 270:468–475
Laudadio G, de Bartolomeu A, Verwijlen LMHM et al (2019) Sulfonyl fluoride synthesis through electrochemical oxidative coupling of Thiols and potassium fluoride. J Am Chem Soc 141:11832–11836
Noël T, Cao Y, Laudadio G (2019) The fundamentals behind the use of flow reactors in electrochemistry. Acc Chem Res 52: 2858–2869
Pletcher D, Green RA, Brown RCD (2018) Flow electrolysis cells for the synthetic organic chemistry laboratory. Chem Rev 118:4573–4591
Atobe M, Tateno H, Matsumura Y (2018) Applications of flow microreactors in Electrosynthetic processes. Chem Rev 118:4541–4572
Mitsudo K, Kurimoto Y, Yoshioka K et al (2018) Miniaturization and combinatorial approach in organic electrochemistry. Chem Rev 118:5985–5999
Folgueiras-Amador AA, Wirth T (2017) Perspectives in flow electrochemistry. J Flow Chem 7:94–95
Laudadio G, de Smet W, Struik L et al (2018) Design and application of a modular and scalable electrochemical flow microreactor. J Flow Chem 8:157–165
Couper AM, Pletcher D, Walsh FC (1990) Electrode materials for electrosynthesis. Chem Rev 90:837–865
Francke R, Little RD (2014) Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem Soc Rev 43:2492–2521
Laudadio G, Straathof NJW, Lanting MD et al (2017) An environmentally benign and selective electrochemical oxidation of sulfides and thiols in a continuous-flow microreactor. Green Chem 19:4061–4066
Laudadio G, Barmpoutsis E, Schotten C et al (2019) Sulfonamide synthesis through electrochemical oxidative coupling of amines and Thiols. J Am Chem Soc 141:5664–5668
Cao Y, Noël T (2019) Efficient Electrocatalytic reduction of furfural to Furfuryl alcohol in a microchannel flow reactor. Org Process Res Dev 23:403–408
Pieber B, Gilmore K, Seeberger PH (2017) Integrated flow processing — challenges in continuous multistep synthesis. J Flow Chem 7:129–136
Fleming GS, Beeler AB (2017) Integrated drug discovery in continuous flow. J Flow Chem 7:124–128
Mennen SM, Alhambra C, Allen CL et al (2019) The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Org Process Res Dev 23:1213–1242
Acknowledgements
Y.C. acknowledges support from the China Scholarship Council (CSC). We acknowledge financial support from the Dutch Science Foundation (NWO) for a VIDI grant for T.N. (SensPhotoFlow, No. 14150). A.A.B. and K.T.O. thank the São Paulo Research Foundation for a FAPESP Mobility Grant (2018/08772-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 7779 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, Y., Adriaenssens, B., de A. Bartolomeu, A. et al. Accelerating sulfonyl fluoride synthesis through electrochemical oxidative coupling of thiols and potassium fluoride in flow. J Flow Chem 10, 191–197 (2020). https://doi.org/10.1007/s41981-019-00070-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00070-9