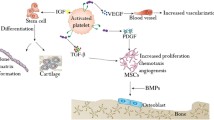
Abstract
Background
Tissue engineering is a branch of medical science research that involves application of biomaterials, to functionally restore and regenerate hard or soft tissue defects of various pathologies and trauma. Biomaterial is a substance, engineered either naturally or artificially, that interacts with the host tissue and produces a desirable outcome. Presently, contending technologies are employed to achieve cost-effective biomaterials. This study aimed to review the applications of platelet concentrates (PCs) as a biomaterial in tissue engineering especially in the field of dentistry and regenerative medicine.
Methods
This review summarizes different types of platelet concentrates (PCs), preparation, biomaterial properties, and applications on stem cell–based therapy.
Results
PCs are fibrin matrix with entrapped platelets and leukocytes that appear as fabricated 3D biomaterials. These naturally derived biomaterials consist of cocktails of growth factors such as platelet-derived growth factor, transforming growth factor beta, vascular endothelial growth factor, epidermal growth factor, insulin-like growth factor-1, and cytokines and chemokines along with fractionated proteins. Hence, they enhance proliferation, migration, angiogenesis, osteogenesis, and chondrogenesis of various types of stem cells.
Conclusion
PCs are easily prepared blood-derived 3D-biomaterials, enriched with numerous growth factors and cytokines. Also they are biocompatible, biodegradable, economical, and autologous with multi-lineage potential, hence advantageous over other synthetic biomaterials. Thus, deeper understanding about the biological potential of PCs provides a new perspective on future direction.
Lay Summary
Tissue engineering is an interdisciplinary approach that involves the use of various methods of engineering as well as life sciences to restore, retain or augment tissue function through application of biomaterials or scaffolds. In this field, biomaterials play crucial role. Use of biomaterials of natural origin such as platelet concentrates are the current trend, since it is autologous, cost effective, non-toxic, biodegradable and easily prepared. Platelet concentrates can be an excellent biomaterial of choice for tissue regeneration and stem cell based therapy.


Similar content being viewed by others
References
Gleadall A, Visscher D, Yang J, Thomas D, Segal J. Review of additive manufactured tissue engineering scaffolds: relationship between geometry and performance. Burns Trauma. 2018;6:1–19.
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011:1–19.
Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue Eng Part B Rev. 2017;23:83–99.
Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–33.
Tsujino T, Masuki H, Nakamura M, Isobe K, Kawabata H, Aizawa H, et al. Striking differences in platelet distribution between advanced-platelet-rich fibrin and concentrated growth factors: effects of silica-containing plastic tubes. J Funct Biomater. 2019;10:43.
Williams DF. Specifications for innovative, enabling biomaterials based on the principles of biocompatibility mechanisms. Front Bioeng Biotechnol. 2019;9(7):255.
Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? Int J Mol Sci. 2019;20:636.
Hortensius RA, Harley BA. Naturally derived biomaterials for addressing inflammation in tissue regeneration. Exp Biol Med (Maywood). 2016;241:1015–24.
Maia FR, Lourenco AH, Granja PL, Goncalves RM, Barrias CC. Effect of cell density on mesenchymal stem cells aggregation in RGD-alginate 3D matrices under osteoinductive conditions. Macromol Biosci. 2014;14:759–71.
Liu X, Peng W, Wang Y, Zhu M, Sun T, Peng Q, et al. Synthesis of an RGD-grafted oxidized sodium alginate-N-succinyl chitosan hydrogel and an in vitro study of endothelial and osteogenic differentiation. J Mater Chem B. 2013;1:4484–92.
Adelow C, Segura T, Hubbell J, Frey P. The effect of enzymatically degradable poly (ethylene glycol) hydrogels on smooth muscle cell phenotype. Biomaterials. 2008;29:314–26.
Hokugo A, Takamoto T, Tabata Y. Preparation of hybrid scaffold fromfibrin and biodegradable polymer fiber. Biomaterials. 2006;27:61–7.
Grad S, Kupcsik L, Gorna K, Gogolewski S, Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24:5163–71.
Munirah S, Kim SH, Ruszymah BHI, Khang G. The use of fibrin and poly (lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: an in vivo analysis. Eur Cells Mater. 2008;15:41–52.
He L, Liu B, Xipeng G, Xie G, Liao S, Quan D, et al. Microstructure and properties of nano-fibrous PCL-b-PLLA scaffolds for cartilage tissue engineering. Eur Cell Mater. 2009;18:63–74.
Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–33.
Parisi L, Toffoli A, Ghiacci G, Macaluso GM. Tailoring the Interface of Biomaterials to Design Effective Scaffolds. J Funct Biomater. 2018;21(9):50.
Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168.
Browning R, Weiser AM, Woolf N, Golish SR, San Giovanni TP, Scuderi GJ, et al. Platelet-rich plasma increases matrix metalloproteinases in cultures of human synovial fibroblasts. J Bone Joint Surg Am. 2012;94:e1721–7.
Ahmed AK, Haylor JL, El Nahas AM, Johnson TS. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int. 2007;71:755–63.
Frank GL, Amir AAM, Stephen MK, Mary ET, Ciara MM, Garry PD, et al. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials. 2010;31:9232–43.
Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a bio-logical scaffold material: structure and function. Acta Biomater. 2009;5:1–13.
Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–24.
Wagoner JAJ, Herschler BA. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011;7:16–30.
Bružauskaitė I, Bironaitė D, Bagdonas E, Bernotienė E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology. 2016;68:355–69.
Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–6.
Hollister SJ. Scaffold engineering: a bridge to where? Biofabrication. 2009;1:012001.
Hosoyama K, Lazurko C, Muñoz M, McTiernan CD, Alarcon EI. Peptide-based functional biomaterials for soft-tissue repair. Front Bioeng Biotechnol. 2019;7:205.
Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23:424–31.
Kingsley CS. Blood coagulation; evidence of an antagonist to factor VI in platelet-rich human plasma. Nature. 1954;173:723–4.
Soffer E, Ouhayoun JP, Anagnostou F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:521–8.
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46.
Choukroun J. Advanced PRF and i-PRF: platelet concentrate or blood concentrate? J Periodontal Med Clin Pract. 2014;1:3.
Everts PA, van Zundert A, Schönberger JP, Devilee RJ, Knape JT. What do we use: platelet-rich plasma or platelet leukocyte gel? J Biomed Mater Res A. 2008;85:1135–6.
Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure plateletrich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27:158–67.
Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13:1185–95.
Tunali M, Özdemir H, Küçükodacı Z, Akman S, Fıratlı E. In vivo evaluation of titanium-prepared platelet-rich fibrin (TPRF): a new platelet concentrate. Br J Oral Maxillofac Surg. 2013;51:438–43.
Mourão CF, Valiense H, Melo ER, Mourão NB, Maia MD. Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note. Rev Col Bras Cir. 2015;42:421–3.
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21.
Pardis H, Hooman K, Saeed R, Ali Dehghani N, Parisa B. Comparative evaluation of conventional and nanosilver-containing leucocyte and platelet-rich fibrin/biomaterial in the anti-biofilm formation of standard species of Candida and Streptococcus. Jundishapur J Microbiol. 2018;11:e68423.
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–89.
Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–44.
Chatterjee A, Debnath K, Ali MM, Babu C, Gowda PL. Comparative histologic evaluation of titanium platelet-rich fibrin and platelet-rich fibrin in hypertensive and smoker participants: a cell cytology study. J Indian Soc Periodontol. 2017;21:195–200.
Chakravarthi S. Platelet rich fibrin in the management of established dry socket. J Korean Assoc Oral Maxillofac Surg. 2017;43:160–5.
Albala DM. Fibrin sealants in clinical practice. Cardiovasc Surg. 2003;11:5–11.
Avanzini MA, Bernardo ME, Cometa AM, Perotti C, Zaffaroni N, Novara F, et al. Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypic and functional comparison of umbilical cord bloodand bone marrow-derived progenitors. Haematologica. 2009;94:1649–60.
Kawamura M, Sawafuji M, Watanabe M, Horinouchi H, Kobayashi K. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg. 2002;73:1098–100.
Joch C. The safety of fibrin sealants. Cardiovasc Surg. 2003;11:23–8.
Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–36.
Aulino P, Costa A, Chiaravalloti E, Perniconi B, Adamo S, Coletti D, et al. Muscle extracellular matrix scaffold is a multipotent environment. Int J Med Sci. 2015;12:336–40.
Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–62.
Escoda Francoli J, Sanchez-Garces MA, Gimeno-Sandig A, Munoz-Guzon F, Barbany-Cairo JR, Badiella-Busquets L. Guided bone regeneration using beta-tricalcium phosphate with and without fibronectin; an experimental study in rats. Clin Oral Implants Res. 2018;29:1038–49.
Anitua E, Muruzabal F, Orive G. Antimicrobial properties of plasma rich in growth factors (Prgf-Endor-Est). Science against microbial pathogens. A. In: Méndez-Vilas, editor. Formatex; 2011. p. 414–21.
Shariati A, Moradabadi A, Azimi T, Ghaznavi-Rad E. Wound healing properties and antimicrobial activity of platelet-derived biomaterials. Sci Rep. 2020;10:1032.
Darouiche RO. Treatment of infections associated with surgical implants. NEngl JMed. 2004;350:1422–9.
Jasmine S, Annamalai T, Janarthanan K, Krishnamoorthy R, Alshatwi AA. Antimicrobial and antibiofilm potential of injectable platelet rich fibrin-a second-generation platelet concentrate-against biofilm producing oral staphylococcus isolates. Saudi J Biol Sci. 2020;27:41–6.
Lorenzo D, Monica B, Christian V, Carlo LR, Silvio T, Massimo DF. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: an in vitro study. PLoS One. 2014;9:e107813.
Khalafi RS, Bradford DW, Wilson MG. Topical application of autologous blood products during surgical closure following a coronary artery bypass graft. Eur J Cardiothorac Surg. 2008;34:360–4.
Yang KC, Wang CH, Chang HH, Chan WP, Chi CH, Kuo TF. Fibrin glue mixed with platelet-rich fibrin as a scaffold seeded with dental bud cells for tooth regeneration. J Tissue Eng Regen Med. 2012;6:777–85.
Clémence T, Marc M, Christophe C, Thierry T, Pascal R, Sophie G, et al. Organic glues or fibrin glues from pooled plasma: efficacy, safety and potential as scaffold delivery systems. J Pharm Pharmaceut Sci. 2012;15:124–40.
Shiga Y, Kubota G, Orita S, Inage K, Kamoda H, Yamashita M, et al. Freeze-dried human platelet-rich plasma retains activation and growth factor expression after an eight-week preservation period. Asian Spine J. 2017;11:329–36.
Herrmann M, Binder A, Menzel U, Zeiter S, Alini M, Verrier S. CD34/CD133 enriched bone marrow progenitor cells promote neovascularization of tissue engineered constructs in vivo. Stem Cell Res. 2014;13:465–77.
Bernardi M, Albiero E, Alghisi A, Chieregato K, Lievore C, Madeo D, et al. Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2013;15:920–9.
Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;13(10):23.
Landesberg R, Glickman RS, Ray M. Quantification of growth factor levels using a simplified method of platelet rich plasma gel preparation. J Oral Maxillofac Surg. 2002;58:297–300.
Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie. 2001;42:55–62.
Garcia-Martinez O, Reyes-Botella C, Diaz-Rodriguez L, EDe Luna B, Ramos Torrecillas B, Vallecillo Capilla MF, et al. Effect of platelet-rich plasma on growth and antigenic profile of human osteoblasts and its clinical impact. Journal of oral and maxillofacial surgery. J Am Assoc Oral Maxillof Surg. 2012;70:1558–64.
Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127:2381–90.
Rousou J, Levitsky S, Gonzalez-Lavin L, Cosgrove D, Magilligan D, Weldon C, et al. Randomized clinical trial of fibrin sealant in patients undergoing resternotomy or reoperation after cardiac operations. A multicenter study. J Thorac Cardiovasc Surg. 1989;97:194–203.
Cakmak O, Babakurban ST, Akkuzu HG, Bilgi S, Ovalı E, Kongur M, et al. Injectable tissue-engineered cartilage using commercially available fibrin glue. Laryngoscope. 2013;123:2986–92.
Brown DM, Barton BR, Young VL, Pruitt BA. Decreased wound contraction with fibrin glue-treated skin grafts. Arch Surg. 1992;127:404–6.
René HF, Alexander HPP, Karl SG, Heinz R. Use of fibrin sealant (Tisseel/Tissucol) in hernia repair: a systematic review. Surg Endosc. 2012;26:1803–12.
Tavares K, Mayo J, Bogenberger K, Davis SS Jr, Yheulon C. Fibrin versus cyanoacrylate glue for fixation in laparoscopic inguinal hernia repair: a network meta-analysis and indirect comparison. Hernia. 2019;10:1007.
Lee JC, Lee SY, Min HJ, et al. Synovium-derived mesenchymal stem cells encapsulated in a novel injectable gel can repair osteochondral defects in a rabbit model. Tissue Eng A. 2012;18:19–20.
Ehrbar M, Metters A, Zammaretti P, Hubbell JA, Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release. 2005;101:93–109.
Tiago MF, Cristina B, Costanza E, Paul A, De B, Giordano P. Platelet lysate gel and endothelial progenitors stimulate microvascular network formation in vitro: tissue engineering implications. Sci Rep. 2016;6:25326.
Abuarqoub DA, Aslam N, Barham RB, et al. The effect of platelet lysate in culture of PDLSCs: an in vitro comparative study. PeerJ. 2019;7:e7465.8.
Kanno T, Takahashi T. Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg. 2005;63:362–9.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Chawla S. Split face comparative study of micro needling with PRP in treating atropic post acne scars. J Cutan Aesthet Surg. 2014;7:209.
Zhu X, Lee L, Jackson J, Tong Y, Wang C. Characterization of porous poly (D, L-lactic-co-glycolic acid) sponges fabricated by supercritical CO2 gas-foaming method as a scaffold for three-dimensional growth of Hep3B cells. Biotechnol Bioeng. 2008;100:998–1009.
Blum IR. Contemporary views on dry socket (alveolar osteitis): a clinical appraisal of standardization, aetiopathogenesis andmanagement: a critical review. Int J Oral Maxillofac Surg. 2002;31:309–17.
Vezeau PJ. Dental extraction wound management: Medicating postextraction sockets. J Oral Maxillofac Surg. 2000;58:531–7.
Anwandter A, Bohmann S, Nally M, Castro AB, Quirynen M, Pinto N. Dimensional changes of the post extraction alveolar ridge, preserved with leukocyte- and platelet rich fibrin: a clinical pilot study. J Dent. 2016;52:23–9.
Woo SM, Kim WJ, Lim HS, Choi NK, Kim SH, Kim SM, et al. Combination of mineral trioxide aggregate and platelet-rich fibrin promotes the odontoblastic differentiation and mineralization of human dental pulp cells via BMP/Smad signaling pathway. J Endod. 2016;42:82–8.
Hoaglin DR, Lines GK. Prevention of localized osteitis in mandibular third-molar sites using platelet-rich fibrin. Int J Dent. 2013;875380.
Borie E, Oliví DG, Orsi IA, Garlet K, Weber B, Beltrán V, et al. Platelet-rich fibrin application in dentistry: a literature review. Int J Clin Exp Med. 2015;8:7922–9.
Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Scacco S, Inchingolo AD, et al. Trial with platelet-rich fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. Eur Rev Med Pharmacol Sci. 2010;14:1075–84.
Bilimoria R, Young H, Patel D, Kwok J. The role of piezoelectric surgery and platelet-rich fibrin in treatment of ORN and MRONJ: a clinical case series. Oral Surg. 2018;11:136–43.
Zhang Z, Li X, Zhao J, Jia W, Wang Z. Effect of autogenous growth factors released from platelet concentrates on the osteogenic differentiation of periodontal ligament fibroblasts: a comparative study. PeerJ. 2019;7:e7984.
Naik B, Karunakar P, Jayadev M, Marshal VR. Role of platelet rich fibrin in wound healing: a critical review. J Conserv Dent. 2013;16:284–93.
Jin Woo K, Sun Jong K, Myung RK. Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: a prospective feasibility study. Br J Oral Maxillofac Surg. 2014;52:854–9.
Ozgul O, Senses F, Er N, Tekin U, Tuz HH, Alkan A, et al. Efficacy of platelet rich fibrin in the reduction of the pain and swelling after impacted third molar surgery: randomized multicenter split-mouth clinical trial. Head Face Med. 2015;11:37.
Del Corso M, Vervelle A, Simonpieri A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: periodontal and dentoalveolar surgery. Curr Pharm Biotechnol. 2012;13:1207–30.
Narang I, Mittal N, Mishra NA. Comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: a clinical study. Contemp Clin Dent. 2015;6:63–8.
Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36:1628–32.
Liao HT, Marra KG, Rubin JP. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20:267–76.
Chignon-Sicard B, Georgiou CA, Fontas E, David S, Dumas P, Ihrai T, et al. Efficacy of leukocyte- and plateletrich fibrin in wound healing: a randomized controlled clinical trial. Plast Reconstr Surg. 2012;130:819e–29e.
Yu P, Zhai Z, Jin X, Yang X, Qi Z. Clinical application of platelet-rich fibrin in plastic and reconstructive surgery: a systematic review. Aesth Plast Surg. 2018;42:511–9.
Sumida R, Maeda T, Kawahara I, Yusa J, Kato Y. Platelet-rich fibrin increases the osteoprotegerin/receptor activator of nuclear factor-κB ligand ratio in osteoblasts. Exp Ther Med. 2019;18:358–65.
Navarro LB, Barchiki F, Navarro JW, et al. Assessment of platelet-rich fibrin in the maintenance and recovery of cell viability of the periodontal ligament. Sci Rep. 2019;9:19476.
Wang X, Yang Y, Zhang Y, Miron RJ. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet-rich plasma. J Cosmet Dermatol. 2019;18:2004–10.
Strauss FJ, Nasirzade J, Kargarpoor Z, Stähli A, Gruber R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: a systematic review of in vitro studies. Clin Oral Investig. 2020;24:569–84.
Chang IC, Tsai CH, Chang YC. Platelet-rich fibrin modulates the expression of extracellular signal-regulated protein kinase and osteoprotegerin in human osteoblasts. J Biomed Mater Res A. 2010;95:327–32.
Wang Z, Han L, Sun T, Wang W, Li X, Wu B. Preparation and effect of lyophilized platelet-rich fibrin on the osteogenic potential of bone marrow mesenchymal stem cells in vitro and in vivo. Heliyon. 2019;5:e02739.
Rozario T, DeSimone DW, et al. Dev Biol. 2010;341:126–40.
Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353–60.
Walid AA. Evaluation of bone regenerative capacity in rats claverial bone defect using platelet rich fibrin with and without beta tri calcium phosphate bone graft material. Saudi Dent J. 2016;28:109–17.
Bruekers SM. Jaspers M, Hendriks JM, Kurniawan NA, Koenderink GH, Kouwe, P H, Huck TSW. Fibrin-fiber architecture influences cell spreading and differentiation. Cell Adhes Migr. 2016;10:495–504.
Kobayashi M, Kawase T, Okuda K, Wolff LF, Yoshie H. In vitro immunological and biological evaluations of the angiogenic potential of platelet-rich fibrin preparations: a standardized comparison with PRP preparations. Int J Implant Dent. 2015;1:31.
Ramaswamy Reddy SH, Reddy R, Babu NC, Ashok GN. Stem-cell therapy and platelet-rich plasma in regenerative medicines: a review on pros and cons of the technologies. J Oral Maxillofac Pathol. 2018;22:367–74.
Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16:204.
Lu HH, Vo JM, Chin HS, Lin J, Cozin M, Tsay R, et al. Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. J Biomed Mater Res A. 2008;86:1128–36.
Author information
Authors and Affiliations
Contributions
SJ: manuscript writing, conception, design, and final approval of manuscript; AT: manuscript writing and corrections; KR: manuscript writing; and AA: manuscript writing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
Ethical approval is not applicable to this article as no data were generated from human/animal during the current study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jasmine, S., Thangavelu, A., Krishnamoorthy, R. et al. Platelet Concentrates as Biomaterials in Tissue Engineering: a Review. Regen. Eng. Transl. Med. 7, 419–431 (2021). https://doi.org/10.1007/s40883-020-00165-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-020-00165-z