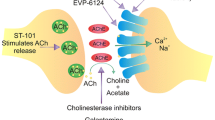
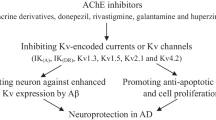
Abstract
Neuroinflammation and cholinergic dysfunction, leading to cognitive impairment, are hallmarks of aging and neurodegenerative disorders, including Alzheimer’s disease (AD). Acetylcholinesterase inhibitors (AChEI), the symptomatic therapy in AD, attenuate and delay the cognitive deficit by enhancing cholinergic synapses. The α7 nicotinic acetylcholine (ACh) receptor has shown a double-edged sword feature, as it binds with high affinity Aβ1–42, promoting intracellular accumulation and Aβ-induced tau phosphorylation, but also exerts neuroprotection by stimulating anti-apoptotic pathways. Moreover, it mediates peripheral and central anti-inflammatory response, being the effector player of the activation of the cholinergic anti-inflammatory pathway (CAIP), that, by decreasing the release of TNF-α, IL-1β, and IL-6, it may have a role in improving cognition. The finding in preclinical models that, in addition to their major function (choline esterase inhibition) AChEIs have neuroprotective properties mediated via α7nAChR and modulate innate immunity, possibly as a result of the increased availability of acetylcholine activating the CAIP, pave the way for new pharmacological intervention in AD and other neurological disorders that are characterized by neuroinflammation. CHRFAM7A is a human-specific gene acting as a dominant negative inhibitor of α7nAChR function, also suggesting a role in affecting human cognition and memory by altering α7nAChR activities in the central nervous system (CNS). This review will summarize the current knowledge on the cholinergic anti-inflammatory pathway in aging-related disorders, and will argue that the presence of the human-restricted CHRFAM7A gene might play a fundamental role in the regulation of CAIP and in the response to AChEI.



Similar content being viewed by others
References
Niraula A, Sheridan JF, Godbout JP (2017) Microglia priming with aging and stress. Neuropsychopharmacology 42:318–333. https://doi.org/10.1038/npp.2016.185
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221:555–563. https://doi.org/10.1016/j.bbr.2010.11.058
Heneka MT, Carson MJ, El Khoury J et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Sinkus ML, Graw S, Freedman R (2015) The human CHRNA7 and CHRFAM7A genes: a review of the genetics, regulation, and function. Neuropharmacology 96:274–288. https://doi.org/10.1016/j.neuropharm.2015.02.006
Tracey KJ (2002) The inflammatory reflex. Nature 420:853. https://doi.org/10.1038/nature01321
Confaloni A, Tosto G, Tata AM (2016) Promising therapies for Alzheimer’s disease. Curr Pharm Des 22:2050–2056
Hoskin JL, Al-Hasan Y, Sabbagh MN (2019) Nicotinic acetylcholine receptor agonists for the treatment of Alzheimer’s dementia: an update. Nicotine Tob Res 21:370–376. https://doi.org/10.1093/ntr/nty116
Akaike A, Takada-Takatori Y, Kume T et al (2010) Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci 40:211–216. https://doi.org/10.1007/s12031-009-9236-1
Pavlov VA, Parrish WR, Rosas-Ballina M et al (2009) Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23:41–45. https://doi.org/10.1016/j.bbi.2008.06.011
Pohanka M (2014) Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int J Mol Sci 15:9809–9825. https://doi.org/10.3390/ijms15069809
Reale M, Iarlori C, Gambi F et al (2004) Treatment with an acetylcholinesterase inhibitor in Alzheimer patients modulates the expression and production of the pro-inflammatory and anti-inflammatory cytokines. J Neuroimmunol 148:162–171. https://doi.org/10.1016/j.jneuroim.2003.11.003
Counts SE, He B, Che S et al (2007) Alpha7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol 64:1771–1776. https://doi.org/10.1001/archneur.64.12.1771
Carson R, Craig D, McGuinness B et al (2008) Alpha7 nicotinic acetylcholine receptor gene and reduced risk of Alzheimer’s disease. J Med Genet 45:244–248. https://doi.org/10.1136/jmg.2007.052704
Chu LW, Ma ES, Lam KK et al (2005) Increased alpha 7 nicotinic acetylcholine receptor protein levels in Alzheimer’s disease patients. Dement Geriatr Cogn Disord 19:106–112. https://doi.org/10.1159/000082661
Weng PH, Chen JH, Chen TF et al (2013) CHRNA7 polymorphisms and response to cholinesterase inhibitors in Alzheimer’s disease. PLoS ONE 8:e84059. https://doi.org/10.1371/journal.pone.0084059
Lasala M, Corradi J, Bruzzone A et al (2018) A human-specific, truncated alpha7 nicotinic receptor subunit assembles with full-length alpha7 and forms functional receptors with different stoichiometries. J Biol Chem 293:10707–10717. https://doi.org/10.1074/jbc.RA117.001698
de Lucas-Cerrillo AM, Maldifassi MC, Arnalich F et al (2011) Function of partially duplicated human α77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J Biol Chem 286:594–606. https://doi.org/10.1074/jbc.M110.180067
Costantini TW, Chan TW, Cohen O et al (2019) Uniquely human CHRFAM7A gene increases the hematopoietic stem cell reservoir in mice and amplifies their inflammatory response. Proc Natl Acad Sci USA 116:7932–7940. https://doi.org/10.1073/pnas.1821853116
Maldifassi MC, Martín-Sánchez C, Atienza G et al (2018) Interaction of the α7-nicotinic subunit with its human-specific duplicated dupα7 isoform in mammalian cells: relevance in human inflammatory responses. J Biol Chem 293:13874–13888. https://doi.org/10.1074/jbc.RA118.003443
Dang X, Eliceiri BP, Baird A et al (2015) CHRFAM7A: a human-specific α7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J 29:2292–2302. https://doi.org/10.1096/fj.14-268037
Borovikova LV, Ivanova S, Zhang M et al (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462. https://doi.org/10.1038/35013070
Hoover DB (2017) Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther 179:1–16. https://doi.org/10.1016/j.pharmthera.2017.05.002
Eglen RM (2012) Overview of muscarinic receptor subtypes. Handb Exp Pharmacol 208:3–28. https://doi.org/10.1007/978-3-642-23274-9_1
Albuquerque EX, Pereira EF, Alkondon M et al (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120. https://doi.org/10.1152/physrev.00015.2008
Zoli M, Pucci S, Vilella A et al (2018) Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr Neuropharmacol 16:338–349. https://doi.org/10.2174/1570159X15666170912110450
Wang H, Yu M, Ochani M et al (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388. https://doi.org/10.1038/nature01339
Eduardo CC, Alejandra TG, Guadalupe DKJ (2019) Modulation of the extraneuronal cholinergic system on main innate response leukocytes. J Neuroimmunol 327:22–35. https://doi.org/10.1016/j.jneuroim.2019.01.008
Kabbani N, Nichols RA (2018) Beyond the channel: metabotropic signaling by nicotinic receptors. Trends Pharmacol Sci 39:354–366. https://doi.org/10.1016/j.tips.2018.01.002
Chang KT, Berg DK (2001) Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron 32:855–865
Berg DK, Conroy WG (2002) Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol 53:512–523. https://doi.org/10.1002/neu.10116
Sharma G, Vijayaraghavan S (2002) Nicotinic receptor signaling in nonexcitable cells. J Neurobiol 53:524–534. https://doi.org/10.1002/neu.10114
Shytle RD, Mori T, Townsend K et al (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89:337–343. https://doi.org/10.1046/j.1471-4159.2004.02347.x
King JR, Gillevet TC, Kabbani N (2017) A G protein-coupled alpha7 nicotinic receptor regulates signaling and TNF-alpha release in microglia. FEBS Open Bio 7:1350–1361. https://doi.org/10.1002/2211-5463.12270
Suzuki T, Hide I, Matsubara A et al (2006) Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res 83:1461–1470. https://doi.org/10.1002/jnr.20850
Richter K, Sagawe S, Hecker A et al (2018) C-reactive protein stimulates nicotinic acetylcholine receptors to control ATP-mediated monocytic inflammasome activation. Front Immunol 9:1604. https://doi.org/10.3389/fimmu.2018.01604
Razani-Boroujerdi S, Boyd RT, Dávila-García MI et al (2007) T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol 179:2889–2898. https://doi.org/10.4049/jimmunol.179.5.2889
Stokes C, Treinin M, Papke RL (2015) Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol Sci 36:514–523. https://doi.org/10.1016/j.tips.2015.05.002
Marrero MB, Bencherif M (2009) Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res 1256:1–7. https://doi.org/10.1016/j.brainres.2008.11.053
de Jonge WJ, van der Zanden EP, The FO et al (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6:844–851. https://doi.org/10.1038/ni1229
Shaw S, Bencherif M, Marrero MB (2002) Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1-42) amyloid. J Biol Chem 277:44920–44924. https://doi.org/10.1074/jbc.M204610200
Yoshikawa H, Kurokawa M, Ozaki N et al (2006) Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol 146:116–123. https://doi.org/10.1111/j.1365-2249.2006.03169.x
Patel H, McIntire J, Ryan S et al (2017) Anti-inflammatory effects of astroglial alpha7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-kappaB pathway and activation of the Nrf2 pathway. J Neuroinflammation 14:192. https://doi.org/10.1186/s12974-017-0967-6
Sun Y, Li Q, Gui H et al (2013) MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res 23:1270–1283. https://doi.org/10.1038/cr.2013.116
Maldifassi MC, Atienza G, Arnalich F et al (2014) A new IRAK-M-mediated mechanism implicated in the anti-inflammatory effect of nicotine via α7 nicotinic receptors in human macrophages. PLoS ONE 9:e108397. https://doi.org/10.1371/journal.pone.0108397
Takahashi T, Morrow JD, Wang H et al (2006) Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem 281:37117–37129. https://doi.org/10.1074/jbc.M608202200
Neri M, Bonassi S, Russo P (2012) Genetic variations in CHRNA7 or CHRFAM7 and susceptibility to dementia. Curr Drug Targets 13:636–643
Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB (2000) beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem 275:5626–5632. https://doi.org/10.1074/jbc.275.8.5626
Dziewczapolski G, Glogowski CM, Masliah E et al (2009) Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. J Neurosci 29:8805–8815. https://doi.org/10.1523/JNEUROSCI.6159-08.2009
Nagele RG, D’Andrea MR, Anderson WJ et al (2002) Intracellular accumulation of beta-amyloid(1-42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience 110:199–211
Wang HY, Li W, Benedetti NJ et al (2003) Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. J Biol Chem 278:31547–31553. https://doi.org/10.1074/jbc.M212532200
Pettit DL, Shao Z, Yakel JL (2001) beta-Amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci 21:120
Park HJ, Lee PH, Ahn YW et al (2007) Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci 26:79–89. https://doi.org/10.1111/j.1460-9568.2007.05636.x
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477. https://doi.org/10.1038/nri3705
Egea J, Buendia I, Parada E et al (2015) Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol 97:463–472. https://doi.org/10.1016/j.bcp.2015.07.032
De Simone R, Ajmone-Cat MA, Carnevale D et al (2005) Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation 2:4. https://doi.org/10.1186/1742-2094-2-4
Benfante R, Antonini RA, De Pizzol M et al (2011) Expression of the α7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J Neuroimmunol 230:74–84. https://doi.org/10.1016/j.jneuroim.2010.09.008
Villiger Y, Szanto I, Jaconi S et al (2002) Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J Neuroimmunol 126:86–98
Maroli A, Di Lascio S, Drufuca L et al (2019) Effect of donepezil on the expression and responsiveness to LPS of CHRNA7 and CHRFAM7A in macrophages: a possible link to the cholinergic anti-inflammatory pathway. J Neuroimmunol 332:155–166. https://doi.org/10.1016/j.jneuroim.2019.04.012
Yasui DH, Scoles HA, Horike S, Meguro-Horike M, Dunaway KW, Schroeder DI, Lasalle JM (2011) 15q11.2-13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum Mol Genet 20:4311–4323. https://doi.org/10.1093/hmg/ddr357
Kunii Y, Zhang W, Xu Q et al (2015) CHRNA7 and CHRFAM7A mRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am J Psychiatry 172:1122–1130. https://doi.org/10.1176/appi.ajp.2015.14080978
Mucchietto V, Fasoli F, Pucci S et al (2018) α9- and α7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line. Br J Pharmacol 175:1957–1972. https://doi.org/10.1111/bph.13954
Araud T, Graw S, Berger R et al (2011) The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7*nAChR function. Biochem Pharmacol 82:904–914. https://doi.org/10.1016/j.bcp.2011.06.018
Wang Y, Xiao C, Indersmitten T et al (2014) The duplicated α7 subunits assemble and form functional nicotinic receptors with the full-length α7. J Biol Chem 289:26451–26463. https://doi.org/10.1074/jbc.M114.582858
Rozycka A, Dorszewska J, Steinborn B et al (2013) A transcript coding for a partially duplicated form of α7 nicotinic acetylcholine receptor is absent from the CD4+ T-lymphocytes of patients with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). Folia Neuropathol 51:65–75
Swaminathan S, Kim S, Shen L et al (2011) Genomic copy number analysis in Alzheimer’s disease and mild cognitive impairment: an ADNI study. Int J Alzheimers Dis 2011:729478. https://doi.org/10.4061/2011/729478
Fehér A, Juhász A, Rimanóczy A et al (2009) Association between a genetic variant of the alpha-7 nicotinic acetylcholine receptor subunit and four types of dementia. Dement Geriatr Cogn Disord 28:56–62. https://doi.org/10.1159/000230036
Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80:844–866. https://doi.org/10.1016/j.neuron.2013.10.008
Ramos FM, Delgado-Vélez M, Ortiz Á et al (2016) Expression of CHRFAM7A and CHRNA7 in neuronal cells and postmortem brain of HIV-infected patients: considerations for HIV-associated neurocognitive disorder. J Neurovirol 22:327–335. https://doi.org/10.1007/s13365-015-0401-8
Costantini TW, Dang X, Coimbra R et al (2015) CHRFAM7A, a human-specific and partially duplicated α7-nicotinic acetylcholine receptor gene with the potential to specify a human-specific inflammatory response to injury. J Leukoc Biol 97:247–257. https://doi.org/10.1189/jlb.4RU0814-381R
Baird A, Coimbra R, Dang X et al (2016) Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease. BBA Clin 5:66–71. https://doi.org/10.1016/j.bbacli.2015.12.003
Colovic MB, Krstic DZ, Lazarevic-Pasti TD et al (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11:315–335. https://doi.org/10.2174/1570159X11311030006
Han SH, Park JC, Byun MS et al (2019) Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol Aging 73:21–29. https://doi.org/10.1016/j.neurobiolaging.2018.09.001
Fodero LR, Mok SS, Losic D et al (2004) Alpha7-nicotinic acetylcholine receptors mediate an Abeta(1-42)-induced increase in the level of acetylcholinesterase in primary cortical neurones. J Neurochem 88:1186–1193. https://doi.org/10.1046/j.1471-4159.2003.02296.x
Inestrosa NC, Dinamarca MC, Alvarez A (2008) Amyloid-cholinesterase interactions. Implications for Alzheimer’s disease. FEBS J 275:625–632. https://doi.org/10.1111/j.1742-4658.2007.06238.x
Reale M, Di Nicola M, Velluto L et al (2014) Selective acetyl- and butyrylcholinesterase inhibitors reduce amyloid-β ex vivo activation of peripheral chemo-cytokines from Alzheimer’s disease subjects: exploring the cholinergic anti-inflammatory pathway. Curr Alzheimer Res 11:608–622
Clarelli F, Mascia E, Santangelo R et al (2016) CHRNA7 gene and response to cholinesterase inhibitors in an Italian cohort of Alzheimer’s disease patients. J Alzheimers Dis 52:1203–1208. https://doi.org/10.3233/JAD-160074
Braga IL, Silva PN, Furuya TK et al (2015) Effect of APOE and CHRNA7 genotypes on the cognitive response to cholinesterase inhibitor treatment at different stages of Alzheimer’s disease. Am J Alzheimers Dis Other Demen 30:139–144. https://doi.org/10.1177/1533317514539540
Russo P, Kisialiou A, Moroni R et al (2017) Effect of genetic polymorphisms (SNPs) in CHRNA7 gene on response to acetylcholinesterase inhibitors (AChEI) in patients with Alzheimer’s disease. Curr Drug Targets 18:1179–1190
Tyagi E, Agrawal R, Nath C et al (2010) Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochem Int 56:135–142. https://doi.org/10.1016/j.neuint.2009.09.011
Noh MY, Koh SH, Kim SM et al (2013) Neuroprotective effects of donepezil against Abeta42-induced neuronal toxicity are mediated through not only enhancing PP2A activity but also regulating GSK-3beta and nAChRs activity. J Neurochem 127:562–574. https://doi.org/10.1111/jnc.12319
Tabet N (2006) Acetylcholinesterase inhibitors for Alzheimer’s disease: anti-inflammatories in acetylcholine clothing! Age Ageing 35:336–338. https://doi.org/10.1093/ageing/afl027
Arias E, Alés E, Gabilan NH et al (2004) Galantamine prevents apoptosis induced by beta-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology 46:103–114
Kim SH, Kandiah N, Hsu JL et al (2017) Beyond symptomatic effects: potential of donepezil as a neuroprotective agent and disease modifier in Alzheimer’s disease. Br J Pharmacol 174:4224–4232. https://doi.org/10.1111/bph.14030
Kimura M, Akasofu S, Ogura H et al (2005) Protective effect of donepezil against Abeta(1-40) neurotoxicity in rat septal neurons. Brain Res 1047:72–84. https://doi.org/10.1016/j.brainres.2005.04.014
Nizri E, Hamra-Amitay Y, Sicsic C et al (2006) Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology 50:540–547. https://doi.org/10.1016/j.neuropharm.2005.10.013
Pollak Y, Gilboa A, Ben-Menachem O et al (2005) Acetylcholinesterase inhibitors reduce brain and blood interleukin-1beta production. Ann Neurol 57:741–745. https://doi.org/10.1002/ana.20454
Reale M, Iarlori C, Gambi F et al (2006) The acetylcholinesterase inhibitor, Donepezil, regulates a Th2 bias in Alzheimer’s disease patients. Neuropharmacology 50:606–613. https://doi.org/10.1016/j.neuropharm.2005.11.006
Conti E, Galimberti G, Tremolizzo L et al (2010) Cholinesterase inhibitor use is associated with increased plasma levels of anti-Abeta 1-42 antibodies in Alzheimer’s disease patients. Neurosci Lett 486:193–196. https://doi.org/10.1016/j.neulet.2010.09.050
Imamura O, Arai M, Dateki M et al (2015) Nicotinic acetylcholine receptors mediate donepezil-induced oligodendrocyte differentiation. J Neurochem 135:1086–1098. https://doi.org/10.1111/jnc.13294
Ludwig J, Höffle-Maas A, Samochocki M et al (2010) Localization by site-directed mutagenesis of a galantamine binding site on α7 nicotinic acetylcholine receptor extracellular domain. J Recept Signal Transduct Res 30:469–483. https://doi.org/10.3109/10799893.2010.505239
Samochocki M, Höffle A, Fehrenbacher A et al (2003) Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 305:1024–1036. https://doi.org/10.1124/jpet.102.045773
Kowal NM, Ahring PK, Liao VWY et al (2018) Galantamine is not a positive allosteric modulator of human alpha4beta2 or alpha7 nicotinic acetylcholine receptors. Br J Pharmacol 175:2911–2925. https://doi.org/10.1111/bph.14329
Giunta B, Ehrhart J, Townsend K et al (2004) Galantamine and nicotine have a synergistic effect on inhibition of microglial activation induced by HIV-1 gp120. Brain Res Bull 64:165–170. https://doi.org/10.1016/j.brainresbull.2004.06.008
Takata K, Kitamura Y, Saeki M et al (2010) Galantamine-induced amyloid-{beta} clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem 285:40180–40191. https://doi.org/10.1074/jbc.M110.142356
Kume T, Sugimoto M, Takada Y et al (2005) Up-regulation of nicotinic acetylcholine receptors by central-type acetylcholinesterase inhibitors in rat cortical neurons. Eur J Pharmacol 527:77–85. https://doi.org/10.1016/j.ejphar.2005.10.028
Takada-Takatori Y, Kume T, Ohgi Y et al (2008) Mechanisms of alpha7-nicotinic receptor up-regulation and sensitization to donepezil induced by chronic donepezil treatment. Eur J Pharmacol 590:150–156. https://doi.org/10.1016/j.ejphar.2008.06.027
Hwang J, Hwang H, Lee HW et al (2010) Microglia signaling as a target of donepezil. Neuropharmacology 58:1122–1129. https://doi.org/10.1016/j.neuropharm.2010.02.003
Arikawa M, Kakinuma Y, Noguchi T et al (2016) Donepezil, an acetylcholinesterase inhibitor, attenuates LPS-induced inflammatory response in murine macrophage cell line RAW 264.7 through inhibition of nuclear factor kappa B translocation. Eur J Pharmacol 789:17–26. https://doi.org/10.1016/j.ejphar.2016.06.053
Wang H, Liao H, Ochani M et al (2004) Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10:1216–1221. https://doi.org/10.1038/nm1124
Cacace R, Sleegers K, Van Broeckhoven C (2016) Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement 12:733–748. https://doi.org/10.1016/j.jalz.2016.01.012
Acknowledgements
We thank Annalisa Maroli for her help in the artworks.
Funding
This work was supported by the National Research Council of Italy (CNR), Research Project Aging: molecular and technological innovations for improving the health of the elderly population (Prot. MIUR 2867 25.11.2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benfante, R., Di Lascio, S., Cardani, S. et al. Acetylcholinesterase inhibitors targeting the cholinergic anti-inflammatory pathway: a new therapeutic perspective in aging-related disorders. Aging Clin Exp Res 33, 823–834 (2021). https://doi.org/10.1007/s40520-019-01359-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01359-4