Abstract
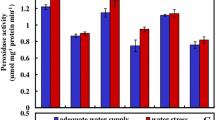
The progressive drying of agricultural fields is considered one of the biggest challenges of food production in the world. The aim of this research was to investigate the effect of the water stress on morphological traits, H2O2, reduced glutathione (GSH) and ascorbic acid (AsA) contents, the activity of APX, GPX, CAT and SOD enzymes and the gene expression of mitochondrial alternative dehydrogenases (NDA2, NDB2, AOX1a and UCP1), in sensitive (Br84-bijelina) and resistant (H301) soybean cultivars. The samples were taken on 0, 1, 3, 6 and 9 days of the experiment. Under the stress conditions, almost all the morphological traits were decreased in both sensitive and resistant cultivars. Unlike sensitive cultivar, the H2O2 content decreased in tolerant cultivar during the stress period. Contents of GSH and AsA were higher under drought stress conditions compared to non-stress treatment in resistant cultivar unlike the sensitive one. Activities of GPX, APX, SOD and CAT were higher in H301 than those in Br84-bijelina cultivar under drought stress conditions. There was an increase in expression of NDA2, NDB2 UCP1 and AOX1a in resistant cultivar compared to sensitive one which indicates ROS scavenging mechanisms of mitochondrial electron transport chain in resistant cultivar act more intensively.



Similar content being viewed by others
References
Abedi, T., Alemzadeh, A., & KazemeIni, S. A. (2010). Effect of organic and inorganic fertilizers on grain yield and protein banding pattern of wheat. Australian Journal of Crop Science, 4, 384–389.
Alekseeva, M., Anischenko, A., Schlegel, I. P., Ott, A. H., & Vorobjeva, L. I. (1983). Improvingcriteria of selection of propioni- bacteria for cheesemaking. In: Shlegel AH (ed) Nauchno-Technichesky Progress. Barnaul, Altaiskii TSNTI, pp. 117–129.
Amako, K., Chen, G. X., & Asada, K. (1994). Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant and Cell Physiology, 35, 497–504.
Anand, A., Gill, H. N., & Trick, B. S. (2003). Stable transgene expression and random gene silencing in wheat. Plant Biotechnology Journal, 1(4), 241–251.
Asada, K. (1999). The water-water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology, 50, 601–640.
Barreto, P., Yassitepe, J. E., Wilson, Z. A., & Arruda, P. (2017). Mitochondrial uncoupling protein 1 overexpression increases yield in Nicotiana tabacum under drought stress by improving source and sink metabolism. Frontiers in Plant Science, 8, 1836.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Bin, W. S., Ping, D. W., Li, Z., Wei, G., Bing, L. J., Gui, P. B., & Feng, C. J. (2012). Evaluation of appropriate reference genes for gene expression studies in pepper by quantitative real-time PCR. Molecular Breeding, 30(3), 1393–1400.
Bokaei, A. S., Babaei, H. R., Habibi, D., Javydfar, F., & Mohammadi, A. (2008). Evaluation of different soybean (Glycine max L.) genotypes under drought stress conditions. Iranian Journal of Agronomy and Plant Breeding, 4(1), 27–38.
Borecký, J., Nogueira, F. T. S., Oliveira, K. A. P., Maia, I. G., Vercesi, A. E., & Arruda, P. (2006). The plant energy-dissipating mitochondrial systems: Depicting the genomic structure and the expression profiles of the gene families of uncoupling protein and alternative oxidase in monocots and dicots. Journal of Experimental Botany, 57, 849–864.
Carneiro, P., Duarte, M., & Videira, A. (2007). Capacity of the alternative pathway in intact plant tissues: Identification of problems and possible. Journal of Molecular Biology, 368, 1114–1121.
Castillo, F. J., & Greppin, H. (1988). Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. leaves after ozone exposure. Environmental and Experimental Botany, 28, 231–238.
Chen, W., Provart, N., Glazebrook, J., Katagiri, F., Chang, H. S., Zou, G., Whitham, S. A., Budworth, P. R., Tao, Y., Xie, Z., et al. (2002). Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. The Plant Cell, 14, 559–574.
Chugh, V., Kaur, N., & Gupta, K. (2011). Evaluation of oxidative stress tolerance in maize (Zea mays L.) seedlings in response to drought. Indian Journal of Biochemistry & Biophysics, 48, 47–53.
Clifton, R., Lister, R., Parker, K. L., Sappl, P. G., Elhafez, D., Millar, A. H., Day, D. A., & Whelan, J. (2005). Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Molecular Biology, 58, 193–212.
Dhindsa, R. S., & Matowe, W. (1981). Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. Journal of Experimental Botany, 32, 79–91.
Dixit, V., Pandey, V., & Shyam, R. (2001). Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum). Journal of Experimental Botany, 52, 1101–1109.
Elhafez, D., Murcha, M. W., Clifton, R., Soole, K. L., Whelan, D. D. A., & J,. (2006). Characterization of mitochondrial alternative NAD(P)H dehydrogenases in Arabidopsis: Intraorganelle location and expression. Plant and Cell Physiology, 47, 43–54.
Faize, M., Burgos, L., Faize, L., Piqueras, A., Nicolas, E., Barba-Espin, G., Clemente-Moreno, M. J., Alcobendas, R., Artlip, T., & Hernandez, J. A. (2011). Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. Journal of Experimental Botany, 62, 2599–2613.
Farooq, M., Wahid, A., Lee, D. J., Cheema, S. A., & Aziz, T. (2010). Comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. Journal of Agronomy and Crop Science, 196, 336–345.
Feierabend, J. (2005). Catalases in plants: Molecular and functional properties and role in stress defense. In N. Smirnoff (Ed.), Antioxidants and reactive oxygen species in plants (pp. 101–140). Blackwell.
Filippou, P., Antoniou, C., & Fotopoulos, V. (2011). Effect of drought and rewatering on the cellular status and antioxidant response of Medicago trunculata plants. Plant Signaling & Behavior, 6, 270–277.
Foyer, C. H. (2018). Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environmental and Experimental Botany, 154, 134–142.
Gallie, D. R. (2013). L-Ascorbic Acid: A multifunctional molecule supporting plant growth and development. Scientifica. https://doi.org/10.1155/2013/795964
Garmash, E. V. (2022). Signal pathways for regulation of plant alternative oxidase genes’ expression. Russian Journal of Plant Physiology, 69(1), 1–16.
Geisler, D. A., Broselid, C., Hederstedt, L., & Rasmusson, A. G. (2007). Ca2+-binding and Ca2+-independent respiratory NADH and NADPH dehydrogenases of Arabidopsis thaliana. Journal of Biological Chemistry, 282(39), 28455–28464.
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide Dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiology, 59, 315–318.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
Granczarska, M. (2005). Response of the ascorbate-glutathione cycle to re-aeration following hypoxia in lupine roots. Plant Physiology and Biochemistry, 43, 583–590.
Gray, G. R., Villarimo, A. R., Whitehead, C. L., & McIntosh, L. (2004). Transgenic tobacco (Nicotiana tabacum L.) plants with increased expression levels of mitochondrial NADP+-dependent isocitrate dehydrogenase: evidence implicating this enzyme in the redox activation of the alternative oxidase. Plant and Cell Physiol, 45(10), 1413–1425.
Habibi, D., Mashdi Akbar Boojar, M., Mahmoudi, A., Ardakani, M. R. & Taleghani, D. (2004). Antioxidative Enzymes in Sunflower Subjected to Drought Stress, 12th Australian Agronomy Conference AAC, 4th ICSC.
Hasheminasab, H., Assad, M. T., Aliakbari, A., & Sahhafi, R. (2012). Influence of drought stress on oxidative damage and antioxidant defense systems in tolerant and sensitive wheat genotypes. Journal of Agricultural Science, 4(8), 20–30.
Ho, L. H., Giraud, E., Uggalla, V., Lister, R., Clifton, R., Glen, A., Thirkettle-Watts, D., Van Aken, O., & Whelan, J. (2008). Identification of regulatory pathways controlling gene expression of stress-responsive mitochondrial proteins in Arabidopsis. Plant Physiology, 147, 1858–1873.
Hu, B. L., Fu, X. Q., Zhang, T., Yong, W. A. N., Xia, L. I., Huang, Y. H., & Xie, J. K. (2011). Genetic analysis on characteristics to measure drought resistance using Dongxiang wild rice (Oryza rufupogon Griff.) and its derived backcross inbred lines population at seedling stage. Agricultural Sciences in China, 10(11), 1653–1664. https://doi.org/10.1016/S1671-2927(11)60164-8
Jimenez, A., Hernández, J. A., del Rio, L. A., & Sevilla, F. (1997). Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiology, 14, 275–284.
Jubany-Marí, T., Munné-Bosch, S., & Alegre, L. (2010). Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiology and Biochemistry, 48, 351–358.
Kausar, R., Hossain, Z., Makino, T., & Komatsu, S. (2012). Characterization of ascorbate peroxidase in soybean under flooding and drought stresses. Molecular Biology Reports, 39(12), 10573–10579.
Keleş, Y., & Öncel, I. (2002). Response of antioxidative defense system to temperature and water stress combinations in wheat seedlings. Plant Science, 163, 783–790.
Khanna-Chopra, R., & Selote, D. S. (2007). Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than-sensitive wheat cultivar under field conditions. Environmental and Experimental Botany, 60, 276–283.
Khurana, E., & Singh, J. (2000). Influence of seed size on seedling growth of Albizia procera under different soil water levels. Annals of Botany, 86, 1185–1192.
Tarumingkeng, R. C., & Coto, Z. (2003). Effects of drought stress on growth and yield of soybean. Kisman. Sci. Philosopy, 702, 798–807.
Krauss, S., Zhang, C. Y., & Lowell, B. B. (2005). The mitochondrial uncoupling-protein homologues. Nature Reviews Molecular Cell Biology, 6, 248–261.
Lawlor, D. W., & Cornic, C. (2002). Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell and Environment, 25, 275–294.
Li, Y. (2008). Kinetics of the antioxidant response to salinity in the halophyte Limonium bicolor. Plant, Soil and Environment, 54, 493–497.
Li, Z., Wakao, S., Fischer, B. B., & Niyogi, K. K. (2009). Sensing and responding to excess light. Annual Review of Plant Biology, 60, 239–260.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT Method. Methods, 25, 402–408.
Lum, M. S., Hanafi, M. M., Rafii, Y. M., & Akmar, A. S. N. (2014). Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. JAPS: Journal of Animal \& Plant Sciences, 24(5), 1487–1493.
McMaster, G. S., & Wilhelm, W. W. (2003). Phenological responses of wheat and barley to water and temperature: Improving simulation models. Journal of Agricultural Science, 141, 129–147.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7, 405–410.
Møller, I. M. (2001). Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology, 52, 561–591.
Møller, I. M., Jensen, P. E., & Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annual Review of Plant Biology, 58, 459–481.
Nakano, Y., & Asada, K. (1987). Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant and Cell Physiology, 28, 131–140.
Nayyar, H., & Gupta, D. (2006). Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environmental and Experimental Botany, 58, 106–113.
Nicholls, N., Gruza, G. V., Jouzel, J., Karl, T. R., Ogallo, L. A., & Parker, D. E., et al. (1996). Observed climate variability and change. In J. T. Houghton (Ed.), Climate change 1995 (pp. 133–192). Cambridge University Press.
Noctor, G., Gomez, L., Vanacker, H., & Foyer, C. H. (2002). Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. Journal of Experimental Botany, 53, 1283–1304.
Ozkur, O., Ozdemir, F., Bor, M., & Turkan, I. (2009). Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environmental and Experimental Botany, 66, 487–492.
Pan, Q. H., Yu, X. C., Zhang, N., Zou, X., Peng, C. C., Wang, X. L., Zou, K. Q., & Zhang, D. P. (2006). Activity, but not expression, of soluble and cell wall-bound acid invertases is induced by abscisic acid in developing apple fruit. Journal of Integrative Plant Biology, 48, 536–549.
Pastore, D., Fratianni, A., Di Pede, S., & Passarella, S. (2000). Effect of fatty acids, nucleotides and reactive oxygen species on durum wheat mitochondria. FEBS Letters, 470, 88–92.
Pinheiro, H. A., DaMatta, F. M., Chaves, A. R. M., Fontes, E. P. B., & Loureiro, M. E. (2004). Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant Science, 167, 1307–1314.
Rabinovich, M. L., Bolobova, A. V., & Vasilchenko, L. G. (2004). Fungal decomposition of natural aromatic structures and xenobiotics: A review. Applied Biochemistry and Microbiology, 40, 1–17.
Rane, J., Maheshwari, M., & Nagarajan, S. (2001). Effect of preanthesis water stress on growth, photosynthesis and yield of six wheat cultivars differing in drought tolerance. Indian Journal of Plant Physiology, 6, 53–60.
Ren, J., Sun, L. N., Zhang, Q. Y., & Song, X. S. (2016). Drought tolerance is correlated with the activity of antioxidant enzymes in Cerasus humilis seedlings. BioMed Research International. https://doi.org/10.1155/2016/9851095
Ribas-Carbo, M., Taylor, N. L., Giles, L., Busquets, S., Finnegan, P. M., Day, D. A., Lambers, H., Medrano, H., Berry, J. A., & Flexas, J. (2005). Effects of water stress on respiration in soybean leaves. Plant Physiology, 139, 466–473.
Rubio, M. C., González, E. M., Minchin, F. R., Webb, K. J., Arrese-Igor, C., Ramos, J., & Becana, M. (2002). Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa overexpressing superoxide dismutases. Physiologia Plantarum, 115, 531–540.
Saha, B., Borovskii, G., & Panda, S. K. (2016). Alternative oxidase and plant stress tolerance. Plant Signaling & Behavior, 11(12), e1256530.
Saika, H., Ohtsu, K., Hamanaka, S., Nakazono, M., Tsutsumi, N., & Hirai, A. (2002). AOX1c, a novel rice gene for alternative oxidase: Comparison with rice AOX1a and AOX1b. Genes & Genetic Systems, 77, 31–38.
Sangtarash, M. H., Qaderi, M. M., Chinnappa, C. C., & Reid, D. M. (2009). Differential sensitivity of canola (Brassica napus L.) seedlings to ultraviolet-B radiation, water stress and abscisic acid. Environ Exper Bot, 66, 212–219.
Sarker, U., & Oba, S. (2018). Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Science and Reports, 8(1), 1–12.
Sharma, P., & Dubey, R. S. (2005). Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regulation, 46, 209–221.
Sharma, P., Jha, A. B., Dubey, R. S., & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012, 1–26.
Silva, M. A., Jifon, J. L., Silva, J. A. G., & Sharma, V. (2007). Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian Journal of Plant Physiology, 19, 193–201.
Sweetman, C., Waterman, C. D., Rainbird, B. M., Smith, P. M., Jenkins, C. D., Day, D. A., & Soole, K. L. (2019). AtNDB2 is the main external NADH dehydrogenase in mitochondria and is important for tolerance to environmental stress. Plant Physiology, 181(2), 774–788.
Szabados, L., & Savouré, A. (2010). Proline: A multifunctional amino acid. Trends in Plant Science, 15, 89–97.
Tahir, M. H. N., & Mehdi, S. S. (2001). Evaluation of open pollinated sunflower (Helianthus annuus L.) population under water stress and normal conditions. International Journal of Agriculture and Biology, 2, 236–238.
Taiz, L., & Zeiger, E. (2006). Plant Physiology (4th ed.). Sinauer Associates Inc.
Taylor, N. L., Day, D. A., & Millar, A. H. (2004). Targets of stress-induced oxidative damage in plant mitochondria and their impact on cell carbon/nitrogen metabolism. J. Exp. Bot, 55(394), 1–10.
Trono, D., Flagella, Z., Laus, M. N., Di Fonzo, N., & Pastore, D. (2004). The uncoupling protein and the potassium channel are activate by hyperosmotic stress in mitochondria from durum wheat seedlings. Plant, Cell and Environ, 27, 437–448.
Tuberosa, R. (2012). Phenotyping for drought tolerance of crops in the genomics era. Frontiers in physiology, 3, 347.
Vernoux, T., Sanchez-Fernandez, R., & May, M. (2002). Glutathione biosynthesis in plants. In D. Inzé & M. V. Montago (Eds.), Oxidative stress in plants (pp. 297–311). Taylor.
Villa-Castorena, M., Ulery, A. L., Catalan-Valencia, E. A., & Rem-menga, M. D. (2003). Salinity and nitrogen rate effects on the growth and yield of chile pepper plants. Soil Science Society of America Journal, 67, 1781–1789.
Walter, J., Nagy, L., Hein, R., Rascher, U., Beierkuhnlein, C., Willner, E., & Jentsch, A. (2011). Do plants remember drought? Hints towards a drought-memory in grasses. Environmental and Experimental Botany, 71, 34–40.
Wang, L. J., Fan, L., Loescher, W., et al. (2010). Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biology, 10, 34–44.
Wang, Z., Li, G., Sun, H., Ma, L., Guo, Y., Zhao, Z., Gao, H., & Mei, L. (2018). Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol Open, 7(11), p.bio 035279.
Webber, M., Barnett, J., Finlayson, B., & Wang, M. (2008). Pricing China’s Irrigation Water. Global Environmental Change, 18(4), 617–625.
Yuan, G. F., Jia, C. G., Li, Z., Sun, B., Zhang, L. P., Liu, N., et al. (2010). Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Scientia Horticulturae, 126, 103–108.
Zhao, R. Z., Jiang, S., Zhang, L., & Yu, Z. B. (2019). Mitochondrial electron transport chain, ROS generation and uncoupling. International Journal of Molecular Medicine, 44(1), 3–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alizadeh, R., Kumleh, H.H. & Rezadoost, M.H. The simultaneous activity of cytosolic and mitochondrial antioxidant mechanisms in neutralizing the effect of drought stress in soybean. Plant Physiol. Rep. 28, 78–91 (2023). https://doi.org/10.1007/s40502-022-00704-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-022-00704-6