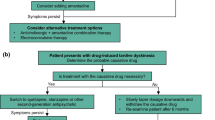
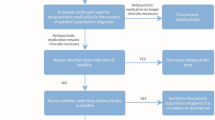
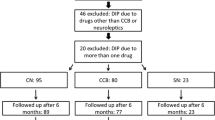
Abstract
Drug-induced parkinsonism (DIP) and tardive dyskinesia (TD) are iatrogenic consequences of antidopaminergic drugs. Both are particularly prevalent among the elderly and those with dementia. However, despite their prevalence, these disorders are often overlooked. Both entities share risk factors, physiopathological mechanisms and, to some degree, therapeutic approaches. Withdrawing the causal agent, reducing the dose or switching to a less potent antidopaminergic drug should be the first therapeutic options. Here we review both entities and emerging therapies including the recently approved drugs deutetrabenazine and valbenazine. We discuss relevant aspects for clinical practice such as new diagnostic techniques and the latest advances in the understanding of DIP and TD.


Similar content being viewed by others
References
Van Gerpen JA. Drug-induced Parkinsonism. Neurologist. 2002;8:363–70. https://doi.org/10.1097/01.nrl.000003012.85777.f1.
Steck H. Extrapyramidal and diencephalic syndrome in the course of largactil and serpasil treatments. Ann Med Psychol. 1954;112:737–44.
Carlsson A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11:490–3.
de la Fuente-Fernandez R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol. 2011;69:803–10. https://doi.org/10.1002/ana.22284.
Caroff SN, Mann SC, Campbell EC, Sullivan KA. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry. 2002;63(Suppl 4):12–9.
Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161:414–25. https://doi.org/10.1176/appi.ajp.161.3.414.
Kane JM. Tardive dyskinesia rates with atypical antipsychotics in adults: prevalence and incidence. J Clin Psychiatry. 2004;65(Suppl 9):16–20.
Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–35. https://doi.org/10.1176/ajp.151.6.825.
de Leon J. The effect of atypical versus typical antipsychotics on tardive dyskinesia: a naturalistic study. Eur Arch Psychiatry Clin Neurosci. 2007;257:169–72. https://doi.org/10.1007/s00406-006-0705-z.
Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21:151–6.
Freyhan FA. Psychomotility and Parkinsonism in treatment with neuroleptic drugs. AMA Arch Neurol Psychiatry. 1957;78:465–72.
Rajput AH, Rozdilsky B, Hornykiewicz O, Shannak K, Lee T, Seeman P. Reversible drug-induced Parkinsonism: clinicopathologic study of two cases. Arch Neurol. 1982;39:644–6.
Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of Parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214.
Llau ME, Nguyen L, Senard JM, Rascol O, Montastruc JL. Drug-induced Parkinson syndrome: 10 years of drug vigilance. Therapie. 1994;49:459–60 (in French).
Erro R, Bhatia KP, Tinazzi M. Parkinsonism following neuroleptic exposure: a double-hit hypothesis? Mov Disord. 2015;30:780–5. https://doi.org/10.1002/mds.26209.
Stephen P, Williamson J. Drug-induced Parkinsonism in the elderly. Lancet. 1984;324:1082–3. https://doi.org/10.1016/S0140-6736(84)91516-2.
López-Sendón JL, Mena MA, de Yébenes JG. Drug-induced Parkinsonism in the elderly. Drugs Aging. 2012;29:105–18.
Seijo-Martinez M, Del Rio MC, Alvarez JR, Prado RS, Salgado ET, Esquete JP, et al. Prevalence of Parkinsonism and Parkinson’s disease in the Arosa Island (Spain): a community-based door-to-door survey. J Neurol Sci. 2011;304:49–54.
De Lau LML, Giesbergen P, De Rijk MC, Hofman A, Koudstaal PJ, Breteler MMB. Incidence of Parkinsonism and Parkinson disease in a general population the Rotterdam Study. Neurology. 2004;63:1240–4.
Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, Porta-Etessam J, Trincado R, Vega S, et al. Incidence of Parkinson disease and Parkinsonism in three elderly populations of central Spain. Neurology. 2004;62:734–41.
Savica R, Grossardt BR, Bower JH, Ahlskog JE, Mielke MM, Rocca WA. Incidence and time trends of drug-induced parkinsonism: a 30-year population-based study. Mov Disord. 2017;32:227–34. https://doi.org/10.1002/mds.26839.
Wenning GK, Kiechl S, Seppi K, Müller J, Högl B, Saletu M, et al. Prevalence of movement disorders in men and women aged 50–89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. 2005;4:815–20. https://doi.org/10.1016/S1474-4422(05)70226-X.
Friedman JH, Trieschmann ME, Fernandez HH. Drug-induced Parkinsonism. In: Factor SA, Lang AE, Weiner WJ, editors. Drug induced movement disorders. Malden: Blackwell Publishing Inc; 2008. p. 103–39. https://doi.org/10.1002/9780470753217.ch6.
Rajput AH, Rozdilsky B, Ang L, Rajput A. Significance of Parkinsonian manifestations in essential tremor. Can J Neurol Sci. 1993;20:114–7.
Kish SJ, Shannak K, Rajput A, Deck JHN, Hornykiewicz O. Aging produces a secific pattern of striatal dopamine loss. Implication for the etiology of idiopathic Parkinson’s disease. J Neurochem. 1992;58:642–8.
Sweet RA, Pollock BG, Rosen J, Mulsant BH, Altieri LP, Perel JM. Early detection of neuroleptic-induced Parkinsonism in elderly patients with dementia. J Geriatr Psychiatry Neurol. 1994;7:251–3. https://doi.org/10.1177/089198879400700411.
Rajput AH, Rajput EF. Octogenarian Parkinsonism–clinicopathological observations. Parkinsonism Relat Disord. 2017;37:50–7. https://doi.org/10.1016/j.parkreldis.2017.01.009.
Rajput AH, Offord KP, Beard CM, Kurland LT. Epidemiology of Parkinsonism: incidence, classification, and mortality. Ann Neurol. 1984;16:278–82. https://doi.org/10.1002/ana.410160303.
Shiroma PR, Geda YE, Mrazek DA. Pharmacogenomic implications of variants of monoaminergic-related genes in geriatric psychiatry. Pharmacogenomics. 2010;11:1305–30. https://doi.org/10.2217/pgs.10.118.
Metzer WS, Newton JEO, Steele RW, Claybrook M, Paige SR, McMillan DE, et al. HLA antigens in drug-induced Parkinsonism. Mov Disord. 1989;4:121–8. https://doi.org/10.1002/mds.870040203.
Assmann BE, Robinson RO, Surtees RAH, Bräutigam C, Heales SJR, Wevers RA, et al. Infantile Parkinsonism-dystonia and elevated dopamine metabolites in CSF. Neurology. 2004;62:1872–4.
Friedman JH. Viewpoint: Challenges in our understanding of neuroleptic induced parkinsonism. Parkinsonism Relat Disord. 2014;20:1325–8. https://doi.org/10.1016/j.parkreldis.2014.09.030.
Yang S-Y, Kao Yang Y-H, Chong M-Y, Yang Y-H, Chang W-H, Lai C-S. Risk of extrapyramidal syndrome in schizophrenic patients treated with antipsychotics: a population-based study. Clin Pharmacol Ther. 2007;81:586–94. https://doi.org/10.1038/sj.clpt.6100069.
Cardoso F, Camargos ST, Silva GA Jr. Etiology of parkinsonism in a Brazilian movement disorders clinic TT [Etiologia de parkinsonismo em uma clínica brasileira de distúrbios do movimento]. Arq Neuropsiquiatr. 1998;56:171–5. https://doi.org/10.1590/S0004-282X1998000200001.
Dall’Igna OP, Tort ABL, Souza DO, Lara DR. Cinnarizine has an atypical antipsychotic profile in animal models of psychosis. J Psychopharmacol. 2005;19:342–6. https://doi.org/10.1177/0269881105053284.
Brücke T, Wöber C, Podreka I, Wöber-Bingöl C, Asenbaum S, Aull S, et al. D2 receptor blockade by flunarizine and cinnarizine explains extrapyramidal side effects. A SPECT study. J Cereb Blood Flow Metab. 1995;15:513–8. https://doi.org/10.1038/jcbfm.1995.63.
Easterford K, Clough P, Kellett M, Fallon K, Duncan S. Reversible Parkinsonism with normal beta-CIT-SPECT in patients exposed to sodium valproate. Neurology. 2004;62:1435–7.
Hawthorne JM, Caley CF. Extrapyramidal reactions associated with serotonergic antidepressants. Ann Pharmacother. 2015;49:1136–52. https://doi.org/10.1177/1060028015594812.
Fernandez HH, Trieschmann ME, Burke MA, Friedman JH. Quetiapine for psychosis in Parkinson’s disease versus dementia with Lewy bodies. J Clin Psychiatry. 2002;63:513–5.
Yaw TK, Fox SH, Lang AE. Clozapine in Parkinsonian rest tremor: a review of outcomes, adverse reactions, and possible mechanisms of action. Mov Disord Clin Pract. 2016;3:116–24. https://doi.org/10.1002/mdc3.12266.
Fernandez HH, Factor SA, Hauser RA, Jimenez-Shahed J, Ondo WG, Jarskog LF, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88:2003–10. https://doi.org/10.1212/wnl.0000000000003960.
Belleau B, Burba J, Pindell M, Reiffenstein J. Effect of deuterium substitution in sympathomimetic amines on adrenergic responses. Science. 1961;133:102–4. https://doi.org/10.1126/science.133.3446.102.
Jankovic J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016;17:2461–70. https://doi.org/10.1080/14656566.2016.1258063.
Kenney C, Hunter C, Jankovic J. Long-term tolerability of tetrabenazine in the treatment of hyperkinetic movement disorders. Mov Disord. 2007;22:193–7.
Hauser RA, Factor SA, Marder SR, Knesevich MA, Ramirez PM, Jimenez R, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174:476–84. https://doi.org/10.1176/appi.ajp.2017.16091037.
Factor SA, Remington G, Comella CL, Correll CU, Burke J, Jimenez R, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017;78:1344–50. https://doi.org/10.4088/JCP.17m11777.
Frank S, Testa CM, Stamler D, Kayson E, Davis C, Edmondson MC, et al. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316:40–50. https://doi.org/10.1001/jama.2016.8655.
Anderson KE, Stamler D, Davis MD, Factor SA, Hauser RA, Isojärvi J, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4:595–604. https://doi.org/10.1016/S2215-0366(17)30236-5.
Rodrigues FB, Duarte GS, Costa J, Ferreira JJ, Wild EJ. Tetrabenazine versus deutetrabenazine for Huntington’s disease: twins or distant cousins? Mov Disord Clin Pract. 2017;4:582–5. https://doi.org/10.1002/mdc3.12483.
Foubert-Samier A, Helmer C, Perez F, Le Goff M, Auriacombe S, Elbaz A, et al. Past exposure to neuroleptic drugs and risk of Parkinson disease in an elderly cohort. Neurology. 2012;79:1615–21. https://doi.org/10.1212/WNL.0b013e31826e25ce.
Chung SJ, Yoo HS, Moon H, Oh JS, Kim JS, Park YH, et al. Early-onset drug-induced Parkinsonism after exposure to offenders implies nigrostriatal dopaminergic dysfunction. J Neurol Neurosurg Psychiatry. 2018;89:169–74. https://doi.org/10.1136/jnnp-2017-315873.
Shuaib UA, Rajput AH, Robinson CA, Rajput A. Neuroleptic-induced Parkinsonism: clinicopathological study. Mov Disord. 2016;31:360–5. https://doi.org/10.1002/mds.26467.
Esper CD, Factor SA. Failure of recognition of drug-induced Parkinsonism in the elderly. Mov Disord. 2008;23:401–4. https://doi.org/10.1002/mds.21854.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601. https://doi.org/10.1002/mds.26424.
Brigo F, Erro R, Marangi A, Bhatia K, Tinazzi M. Differentiating drug-induced Parkinsonism from Parkinson’s disease: an update on non-motor symptoms and investigations. Parkinsonism Relat Disord. 2014;20:808–14. https://doi.org/10.1016/j.parkreldis.2014.05.011.
López-Sendón Moreno JL, Alonso-Cánovas A, Buisán Catevilla J, García Barragán N, Corral Corral I, de Felipe Mimbrera A, et al. Substantia nigra echogenicity predicts response to drug withdrawal in suspected drug-induced Parkinsonism. Mov Disord Clin Pract. 2016;3:268–74. https://doi.org/10.1002/mdc3.12281.
Tinazzi M, Ottaviani S, Isaias IU, Pasquin I, Steinmayr M, Vampini C, et al. [123I]FP–CIT SPET imaging in drug-induced Parkinsonism. Mov Disord. 2008;23:1825–9. https://doi.org/10.1002/mds.22098.
Tinazzi M, Geroin C, Gandolfi M, Smania N, Tamburin S, Morgante F, et al. Pisa syndrome in Parkinson’s disease: an integrated approach from pathophysiology to management. Mov Disord. 2016;31:1785–95. https://doi.org/10.1002/mds.26829.
Cuberas-Borrós G, Lorenzo-Bosquet C, Aguadé-Bruix S, Hernández-Vara J, Pifarré-Montaner P, Miquel F, et al. Quantitative evaluation of striatal I-123-FP-CIT uptake in essential tremor and Parkinsonism. Clin Nucl Med. 2011;36:991–6. https://doi.org/10.1097/RLU.0b013e3182291a7b.
Diaz-Corrales FJ, Sanz-Viedma S, Garcia-Solis D, Escobar-Delgado T, Mir P. Clinical features and 123I-FP-CIT SPECT imaging in drug-induced Parkinsonism and Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2010;37:556–64. https://doi.org/10.1007/s00259-009-1289-4.
Rascol O, Schelosky L. 123I-metaiodobenzylguanidine scintigraphy in Parkinson’s disease and related disorders. Mov Disord. 2009;24:S732–41. https://doi.org/10.1002/mds.22499.
Lee PH, Kim JS, Shin DH, Yoon S-N, Huh K. Cardiac 123I-MIBG scintigraphy in patients with drug induced Parkinsonism. J Neurol Neurosurg Psychiatry. 2006;77:372–4.
Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–45. https://doi.org/10.1016/S1474-4422(06)70373-8.
Chien CP, Dimascio A, Cole JO. Antiparkinsonian agents and depot phenothiazine. Am J Psychiatry. 1974;131:86–90. https://doi.org/10.1176/ajp.131.1.86.
Keepers GA, Clappison VJ, Casey DE. Initial anticholinergic prophylaxis for neuroleptic-induced extrapyramidal syndromes. Arch Gen Psychiatry. 1983;40:1113–7.
Hardie RJ, Lees AJ. Neuroleptic-induced Parkinson’s syndrome: clinical features and results of treatment with levodopa. J Neurol Neurosurg Psychiatry. 1988;51:850–4.
Gerlach J. Relationship between tardive dyskinesia, l-dopa-induced hyperkinesia and Parkinsonism. Psychopharmacology (Berl). 1977;51:259–63.
Saltz BL, Woerner MG, Robinson DG, Kane JM. Side effects of antipsychotic drugs. Avoiding and minimizing their impact in elderly patients. Postgrad Med. 2000;107(169–72):175–8. https://doi.org/10.3810/pgm.2000.02.891.
Ory-Magne F, Corvol J-C, Azulay J-P, Bonnet A-M, Brefel-Courbon C, Damier P, et al. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology. 2014;82:300–7. https://doi.org/10.1212/WNL.0000000000000050.
Wolf E, Seppi K, Katzenschlager R, Hochschorner G, Ransmayr G, Schwingenschuh P, et al. Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Mov Disord. 2010;25:1357–63. https://doi.org/10.1002/mds.23034.
Silver H, Geraisy N, Schwartz M. No difference in the effect of biperiden and amantadine on Parkinsonian- and tardive dyskinesia-type involuntary movements: a double-blind crossover, placebo-controlled study in medicated chronic schizophrenic patients. J Clin Psychiatry. 1995;56:167–70.
Pahwa R, Hauser RA. ADS-5102 (Amantadine) extended release for levodopa-induced dyskinesia. JAMA Neurol. 2017;74:1507–8. https://doi.org/10.1001/jamaneurol.2017.3205.
Tinazzi M, Antonini A, Bovi T, Pasquin I, Steinmayr M, Moretto G, et al. Clinical and [123I]FP-CIT SPET imaging follow-up in patients with drug-induced Parkinsonism. J Neurol. 2009;256:910–5. https://doi.org/10.1007/s00415-009-5039-0.
Goswami U, Dutta S, Kuruvilla K, Papp E, Perenyi A. Electroconvulsive therapy in neuroleptic-induced Parkinsonism. Biol Psychiatry. 1989;26:234–8.
Berg JE. Electroconvulsive treatment of a patient with Parkinson’s disease and moderate depression. Ment Illn. 2011;3:8–10. https://doi.org/10.4081/mi.2011.e3.
Moellentine C, Rummans T, Ahlskog JE, Harmsen WS, Suman VJ, O’Connor MK, et al. Effectiveness of ECT in patients with Parkinsonism. J Neuropsychiatry Clin Neurosci. 1998;10:187–93. https://doi.org/10.1176/jnp.10.2.187.
Aquino CCH, Lang AE. Tardive dyskinesia syndromes: current concepts. Parkinsonism Relat Disord. 2014;20:S113–7. https://doi.org/10.1016/s1353-8020(13)70028-2.
Fahn S, Jankovic J, Hallett M. The tardive syndromes: phenomenology, concepts on pathophysiology and treatment, and other neuroleptic-induced syndromes. Principles and practice of movement disorders. 2nd ed. Philadelphia: Elsevier; 2011. p. 415–46. https://doi.org/10.1016/b978-1-4377-2369-4.00019-6.
Jeste DV, Wyatt RJ. Therapeutic strategies against tardive dyskinesia. Two decades of experience. Arch Gen Psychiatry. 1982;39:803–16.
Kane JM, Smith JM. Tardive dyskinesia: prevalence and risk factors, 1959 to 1979. Arch Gen Psychiatry. 1982;39:473–81.
Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly. Drugs Aging. 2000;17:363–84.
Caligiuri MP, Lacro JP, Rockwell E, McAdams LA, Jeste DV. Incidence and risk factors for severe tardive dyskinesia in older patients. Br J Psychiatry. 1997;171:148–53.
Tarsy D, Baldessarini RJ. Epidemiology of tardive dyskinesia: is risk declining with modern antipsychotics? Mov Disord. 2006;21:589–98. https://doi.org/10.1002/mds.20823.
Carbon M, Hsieh C-H, Kane JM, Correll CU. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78:e264–78. https://doi.org/10.4088/JCP.16r10832.
Woods SW, Morgenstern H, Saksa JR, Walsh BC, Sullivan MC, Money R, et al. Incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications. J Clin Psychiatry. 2010;71:463–74. https://doi.org/10.4088/JCP.07m03890yel.
van Os J, Fahy T, Jones P, Harvey I, Toone B, Murray R. Tardive dyskinesia: who is at risk? Acta Psychiatr Scand. 1997;96:206–16.
van Harten PN, Hoek HW, Matroos GE, Koeter M, Kahn RS. Intermittent neuroleptic treatment and risk for tardive dyskinesia: Curaçao Extrapyramidal Syndromes Study III. Am J Psychiatry. 1998;155:565–7. https://doi.org/10.1176/ajp.155.4.565.
Smith JM, Baldessarini RJ. Changes in prevalence, severity, and recovery in tardive dyskinesia with age. Arch Gen Psychiatry. 1980;37:1368–73.
Woerner MG, Alvir JM, Saltz BL, Lieberman JA, Kane JM. Prospective study of tardive dyskinesia in the elderly: rates and risk factors. Am J Psychiatry. 1998;155:1521–8. https://doi.org/10.1176/ajp.155.11.1521.
Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013. https://doi.org/10.7916/D88P5Z71.
Wonodi I, Adami HM, Cassady SL, Sherr JD, Avila MT, Thaker GK. Ethnicity and the course of tardive dyskinesia in outpatients presenting to the motor disorders clinic at the Maryland psychiatric research center. J Clin Psychopharmacol. 2004;24:592–8.
Tenback DE, van Harten PN, van Os J. Non-therapeutic risk factors for onset of tardive dyskinesia in schizophrenia: a meta-analysis. Mov Disord. 2009;24:2309–15. https://doi.org/10.1002/mds.22707.
Ferentinos P, Dikeos D. Genetic correlates of medical comorbidity associated with schizophrenia and treatment with antipsychotics. Curr Opin Psychiatry. 2012;25:381–90. https://doi.org/10.1097/YCO.0b013e3283568537.
Ganzini L, Casey DE, Hoffman WF, McCall AL. The prevalence of metoclopramide-induced tardive dyskinesia and acute extrapyramidal movement disorders. Arch Intern Med. 1993;153:1469–75.
Dubovsky SL, Thomas M. Tardive dyskinesia associated with fluoxetine. Psychiatr Serv. 1996;47:991–3. https://doi.org/10.1176/ps.47.9.991.
Woogen S, Graham J, Angrist B. A tardive dyskinesia-like syndrome after amitriptyline treatment. J Clin Psychopharmacol. 1981;1:34–6.
Harrison MB, Lyons GR, Landow ER. Phenytoin and dyskinesias: a report of two cases and review of the literature. Mov Disord. 1993;8:19–27. https://doi.org/10.1002/mds.870080104.
Thach BT, Chase TN, Bosma JF. Oral facial dyskinesia accociated with prolonged use of antihistaminic decongestants. N Engl J Med. 1975;293:486–7. https://doi.org/10.1056/NEJM197509042931008.
Raja M, Azzoni A. Tardive dyskinesia after long-term veralipride treatment. J Neuropsychiatry Clin Neurosci. 2005;17:252–3. https://doi.org/10.1176/jnp.17.2.252-a.
Bordia T, McIntosh JM, Quik M. Nicotine reduces antipsychotic-induced orofacial dyskinesia in Rats. J Pharmacol Exp Ther. 2012;340:612–9. https://doi.org/10.1124/jpet.111.189100.
Waddington JL. Spontaneous orofacial movements induced in rodents by very long-term neuroleptic drug administration: phenomenology, pathophysiology and putative relationship to tardive dyskinesia. Psychopharmacology (Berl). 1990;101:431–47.
Blanchet PJ, Parent M-T, Rompré PH, Lévesque D. Relevance of animal models to human tardive dyskinesia. Behav Brain Funct. 2012;8:12. https://doi.org/10.1186/1744-9081-8-12.
Loonen AJM, Ivanova SA. New insights into the mechanism of drug-induced dyskinesia. CNS Spectr. 2013;18:15–20. https://doi.org/10.1017/S1092852912000752.
Lohr JB, Kuczenski R, Niculescu AB. Oxidative mechanisms and tardive dyskinesia. CNS Drugs. 2003;17:47–62.
Elkashef AM, Wyatt RJ. Tardive dyskinesia: possible involvement of free radicals and treatment with vitamin E. Schizophr Bull. 1999;25:731–40.
Kiriakakis V, Bhatia KP, Quinn NP, Marsden CD. The natural history of tardive dystonia. A long-term follow-up study of 107 cases. Brain. 1998;121(Pt 1):2053–66.
Teo JT, Edwards MJ, Bhatia K. Tardive dyskinesia is caused by maladaptive synaptic plasticity: a hypothesis. Mov Disord. 2012;27:1205–15. https://doi.org/10.1002/mds.25107.
Steen VM, Løvlie R, MacEwan T, McCreadie RG. Dopamine D3-receptor gene variant and susceptibility to tardive dyskinesia in schizophrenic patients. Mol Psychiatry. 1997;2:139–45.
Bakker PR, van Harten PN, van Os J. Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: a meta-analysis of pharmacogenetic interactions. Mol Psychiatry. 2008;13:544–56. https://doi.org/10.1038/sj.mp.4002142.
Bordia T, Perez XA, Heiss J, Zhang D, Quik M. Optogenetic activation of striatal cholinergic interneurons regulates l-dopa-induced dyskinesias. Neurobiol Dis. 2016;91:47–58. https://doi.org/10.1016/j.nbd.2016.02.019.
Salem H, Pigott T, Zhang XY, Zeni CP, Teixeira AL. Antipsychotic-induced Tardive dyskinesia: from biological basis to clinical management. Expert Rev Neurother. 2017;17:883–94. https://doi.org/10.1080/14737175.2017.1361322.
Tarsy D. Neuroleptic-induced extrapyramidal reactions: classification, description, and diagnosis. Clin Neuropharmacol. 1983;6(Suppl 1):S9–26.
Tarsy D, Baldessarini RJ. Tardive dyskinesia. Annu Rev Med. 1984;35:605–23. https://doi.org/10.1146/annurev.me.35.020184.003133.
Gardos G, Casey DE, Cole JO, Perenyi A, Kocsis E, Arato M, et al. Ten-year outcome of tardive dyskinesia. Am J Psychiatry. 1994;151:836–41. https://doi.org/10.1176/ajp.151.6.836.
Labbate LA, Lande RG, Jones F, Oleshansky MA. Tardive dyskinesia in older out-patients: a follow-up study. Acta Psychiatr Scand. 1997;96:195–8. https://doi.org/10.1111/j.1600-0447.1997.tb10151.x.
Burke RE, Kang UJ, Jankovic J, Miller LG, Fahn S. Tardive akathisia: an analysis of clinical features and response to open therapeutic trials. Mov Disord. 1989;4:157–75. https://doi.org/10.1002/mds.870040208.
Munetz MR, Roth LH, Cornes CL. Tardive dyskinesia and informed consent: myths and realities. Bull Am Acad Psychiatry Law. 1982;10:77–88.
Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1:193–208. https://doi.org/10.1002/mds.870010305.
Stacy M, Jankovic J. Tardive tremor. Mov Disord. 1992;7:53–7. https://doi.org/10.1002/mds.870070110.
Fountoulakis KN, Samara M, Siapera M, Iacovides A. Tardive Tourette-like syndrome: a systematic review. Int Clin Psychopharmacol. 2011;26:237–42. https://doi.org/10.1097/YIC.0b013e32834aa924.
Tominaga H, Fukuzako H, Izumi K, Koja T, Fukuda T, Fujii H, et al. Tardive myoclonus [letter]. Lancet. 1987;1:322.
Ford B, Greene P, Fahn S. Oral and genital tardive pain syndromes. Neurology. 1994;44:2115–9.
Jankovic J, Casabona J. Coexistent tardive dyskinesia and parkinsonism. Clin Neuropharmacol. 1987;10:511–21.
Quitkin F, Rifkin A, Gochfeld L, Klein DF. Tardive dyskinesia: are first signs reversible? Am J Psychiatry. 1977;134:84–7. https://doi.org/10.1176/ajp.134.1.84.
Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135:1321–4. https://doi.org/10.1176/ajp.135.11.1321.
Bergman H, Rathbone J, Agarwal V, Soares-Weiser K. Antipsychotic reduction and/or cessation and antipsychotics as specific treatments for tardive dyskinesia. Cochrane Database Syst Rev. 2018. https://doi.org/10.1002/14651858.CD000459.pub3.
Fernandez HH, Krupp B, Friedman JH. The course of tardive dyskinesia and parkinsonism in psychiatric inpatients: 14-Year follow-up. Neurology. 2001;56:805–7. https://doi.org/10.1212/WNL.56.6.805.
Emsley R, Turner HJ, Schronen J, Botha K, Smit R, Oosthuizen PP. A single-blind, randomized trial comparing quetiapine and haloperidol in the treatment of tardive dyskinesia. J Clin Psychiatry. 2004;65:696–701.
Bassitt DP, Louzã Neto MR. Clozapine efficacy in tardive dyskinesia in schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:209–11.
Kenney C, Jankovic J. Tetrabenazine in the treatment of hyperkinetic movement disorders. Expert Rev Neurother. 2006;6:7–17. https://doi.org/10.1586/14737175.6.1.7.
Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther. 2012;34:1487–504. https://doi.org/10.1016/j.clinthera.2012.06.010.
Davis MC, Miller BJ, Kalsi JK, Birkner T, Mathis MV. Efficient trial design—FDA approval of valbenazine for tardive dyskinesia. N Engl J Med. 2017;376:2503–6. https://doi.org/10.1056/NEJMp1704898.
Bergman H, Soares-Weiser K. Anticholinergic medication for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2018. https://doi.org/10.1002/14651858.CD000204.pub2.
Bhidayasiri R, Fahn S, Weiner WJ, Gronseth GS, Sullivan KL, Zesiewicz TA. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81:463–9. https://doi.org/10.1212/WNL.0b013e31829d86b6.
Oertel W, Eggert K, Pahwa R, Tanner CM, Hauser RA, Trenkwalder C, et al. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord. 2017;32:1701–9. https://doi.org/10.1002/mds.27131.
Ramirez-Castaneda J, Jankovic J. Long-term efficacy and safety of botulinum toxin injections in dystonia. Toxins (Basel). 2013;5:249–66. https://doi.org/10.3390/toxins5020249.
Spindler MA, Galifianakis NB, Wilkinson JR, Duda JE. Globus pallidus interna deep brain stimulation for tardive dyskinesia: case report and review of the literature. Parkinsonism Relat Disord. 2013;19:141–7. https://doi.org/10.1016/j.parkreldis.2012.09.016.
Quik M, Zhang D, Perez XA, Bordia T. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014;144:50–9. https://doi.org/10.1016/j.pharmthera.2014.05.004.
Bhidayasiri R, Jitkritsadakul O, Friedman JH, Fahn S. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67–75. https://doi.org/10.1016/j.jns.2018.02.010.
Meyer JM. Future directions in tardive dyskinesia research. J Neurol Sci. 2018;389:76–80. https://doi.org/10.1016/j.jns.2018.02.004.
Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
Jose Lopez-Sendón has received honoraria from Zambon and travel grants from Lundbeck and KRKA pharmaceuticals. He has no other conflicts of interest. Carlos Estevez-Fraga and Paul Zeun have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Estevez-Fraga, C., Zeun, P. & López-Sendón Moreno, J.L. Current Methods for the Treatment and Prevention of Drug-Induced Parkinsonism and Tardive Dyskinesia in the Elderly. Drugs Aging 35, 959–971 (2018). https://doi.org/10.1007/s40266-018-0590-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-018-0590-y