Abstract
Elagolix (ORILISSA™), an orally bioavailable, second-generation, non-peptide gonadotropin-releasing hormone (GnRH) receptor antagonist, is being developed AbbVie and Neurocrine Biosciences for the treatment of reproductive hormone-dependent disorders in women. In July 2018, the US FDA approved elagolix tablets for the management of moderate to severe pain associated with endometriosis. This approval was based on positive results in two replicate phase III trials; additional phase III trials in the USA, Canada and Puerto Rico are currently evaluating elagolix as both monotherapy and in combination with low-dose hormone add-back therapy in the same indication. Elagolix with and without low-dose hormone add-back therapy is also undergoing phase III clinical development for heavy menstrual bleeding associated with uterine fibroids in the aforementioned locations. This article summarizes the milestones in the development of elagolix leading to its first approval for the management of moderate to severe pain associated with endometriosis.
Similar content being viewed by others
1 Introduction
Elagolix (ORILISSA™) is a small molecule, second-generation, non-peptide GnRH receptor antagonist that reduces levels of ovarian sex hormones in the blood [1,2,3]. Elagolix is being developed by AbbVie in collaboration with Neurocrine Biosciences as an orally-administered treatment for hormone-dependent proliferative disorders such as endometriosis and uterine leiomyoma.
Elagolix 150 mg and 200 mg tablets were approved by the US FDA on 23 July 2018 for the management of moderate to severe pain associated with endometriosis [4]. Elagolix is the first oral treatment for moderate to severe endometriosis-associated pain to receive FDA approval in over a decade [4]. In patients with normal liver function or mild hepatic impairment (Child-Pugh A) and no co-existing conditions, the recommended dose is 150 mg once daily for ≤ 24 months; in patients with co-existing dyspareunia, 200 mg twice daily for ≤ 6 months should be considered [1]. In patients with moderate hepatic impairment (Child-Pugh B), the recommended dose is 150 mg once daily for ≤ 6 months. Elagolix should be administered at the lowest effective dose and duration of use should be limited to reduce bone loss. Elagolix is contraindicated in women who are pregnant, have known osteoporosis or severe hepatic impairment (Child-Pugh C), or are concomitantly using strong organic anion transporting polypeptide (OATP) 1B1 inhibitors [1].

1.1 Company Agreements
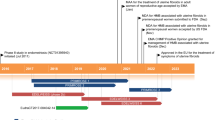
In June 2010, Neurocrine Biosciences and Abbott (now AbbVie) entered into a collaboration agreement to develop and commercialise elagolix for the treatment of endometriosis and uterine leiomyoma [5]. This agreement granted Abbott worldwide exclusive rights to develop and commercialise elagolix and all other next-generation GnRH antagonists developed by Neurocrine Biosciences for women’s and men’s health indications. The terms of the agreement required Abbott to make an upfront payment of US$75 million and provide funding for all ongoing development activities. Furthermore, Neurocrine Biosciences is eligible to receive from Abbott additional payments of ≈ US$500 million for achieving specific development, regulatory and commercial milestones, as well as funding for certain internal collaboration expenses and royalty payments on future product sales [5]. By the end of October 2010, Investigational New Drug applications for elagolix had been transferred from Neurocrine Biosciences to Abbott [6].

Chemical structure of elagolix
Neurocrine Biosciences had entered into an agreement with the Mount Sinai School of Medicine of the City University of New York (Mt. Sinai) in August 1999 [7]. This resulted in Neurocrine Biosciences acquiring a non-exclusive licence to two patents related to screening compounds for GnRH activity. The agreement also allowed Neurocrine Biosciences to grant sub-licences to third parties, subject to the consent of Mt. Sinai. Neurocrine Biosciences paid at upfront fee of US$50,000 and is required to pay an additional annual license fee of US$10,000, as well as a royalty payment of 1% of licensed product net sales. The agreement is to remain in effect until either 15 years from the date of the first commercial sale of the first licensed product, or the expiration of the last of the licensed patents to expire (whichever is later); under certain conditions, the agreement may be terminated earlier [7].
2 Scientific Summary
2.1 Pharmacodynamics
Elagolix is a highly potent (KD = 54 pM [3]), non-peptide GnRH receptor antagonist that binds competitively to GnRH receptors in the pituitary gland [1, 3]. Through blocking endogenous GnRH signalling, elagolix suppresses luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby reducing estradiol and progesterone production [1,2,3].
Elagolix rapidly suppressed LH, FSH, estradiol and progesterone in a dose-dependent manner when administered orally at doses of 150 mg once daily or 100–400 mg twice daily in a clinical trial in healthy, premenopausal women [2]. In women with endometriosis participating in phase III trials, elagolix 150 mg once daily and 200 mg twice daily regimens reduced median estradiol concentrations to ≈ 42 and 12 pg/mL [1]. Elagolix 150 mg once daily and 200 mg twice daily regimens were associated with ovulation rates of ≈ 50 and 32% in healthy women administered elagolix for three menstrual cycles, and transvaginal ultrasounds showed dose-dependent decreases in mean endometrial thickness relative to baseline [1].
Elagolix did not prolong QTc interval to any clinically relevant degree when administered at a supratherapeutic single dose of 1200 mg (producing elagolix concentrations 17-times higher than those observed with the 200 mg twice daily dose) in a thorough QTc study in healthy premenopausal women [1].
The efficacy of elagolix may be reduced by oestrogen-containing contraceptives; women should use non-hormonal contraception while undergoing treatment with elagolix, as well as for 1 week after treatment discontinuation [1].
2.2 Pharmacokinetics
Elagolix had approximately dose-proportional pharmacokinetics in healthy premenopausal women (dose range 100–400 mg twice daily) [2]. Elagolix was rapidly absorbed, with peak plasma concentration (Cmax) reached in a median of ≈ 1 h following oral administration at the recommended doses [2]. Plasma protein binding was 80% [1]. Exposure [area under the plasma concentration-time curve (AUC)] and Cmax were reduced by 24 and 36% when elagolix was administered with a high-fat meal relative to fasting [1]. Minimal accumulation occurred with repeated doses of 150 mg once daily or 200 mg twice daily [2]. The apparent volume of distribution at steady state is 1674 L with elagolix 150 mg once daily and 881 L with elagolix 200 mg twice daily [1].
Elagolix undergoes mostly CYP3A-mediated metabolism, with CYP2D6, CYP2C8 and uridine glucuronosyl transferases (UGTs) having minor roles in metabolizing elagolix [1]. Elagolix has a short terminal phase elimination half-life of 4–6 h [1, 2]. At doses of 150 mg once daily and 200 mg twice daily, oral clearance was 123 and 144 L/h [1]. It is eliminated via hepatic metabolism and the majority of the dose is excreted in the faeces (90%; <3% excreted in the urine) [1].
Elagolix pharmacokinetic properties are not affected to a clinically meaningful extent by body weight, body mass index or race/ethnicity [1, 8]. As the OATP1B1 transporter protein is involved in the disposition of elagolix, higher elagolix plasma concentrations have been observed in patients with two copies of a reduced function allele in the gene encoding OATP1B1 (i.e. the low-frequency SLCO1B1 521C/C genotype) [1]. Renal impairment has no effect on elagolix exposure (AUC and Cmax) [1, 8]. While elagolix exposure in women with mild hepatic impairment did not substantially differ from that in women with normal hepatic function, exposure was ≈ 3- and 7-fold higher in women with moderate or severe hepatic function, respectively [1]. No dose adjustment is required in patients with mild hepatic impairment. In patients with moderate hepatic impairment, the only recommended dosage regimen is 150 mg once daily for ≤ 6 months. Elagolix is contraindicated in patients with severe hepatic impairment [1].
Concomitant use of elagolix and OATP1B1 inhibitors (e.g. rifampin) may increase the plasma concentration of elagolix [1, 9]. Coadministration of elagolix 200 mg twice daily and rifampin is not recommended; if used with rifampin, elagolix should be limited to 150 mg once daily for ≤ 6 months [1]. Coadministration of elagolix and strong OATP1B1 inhibitors (e.g. cyclosporine and gemfibrozil) is contraindicated [1]. Elagolix plasma exposure increased when coadministered with ketoconazole [1, 10]; the coadministration of elagolix 200 mg twice daily and strong CYP3A inhibitors for > 1 month is not recommended, and concomitant use of elagolix 150 mg once daily and strong CYP3A inhibitors should be limited to 6 months [1]. Elagolix increases the plasma exposure of the antiarrhythmic digoxin when coadministered and clinical monitoring for digoxin is thus recommended when these drugs are used concomitantly [1]. As elagolix lowers the plasma exposures of midazolam and rosuvastatin, increased doses of these agents may be warranted when coadministered with elagolix [1].
Features and properties of elagolix
Alternative names | ABT-620; elagolix sodium, NBI-56418; ORILISSA |
Class | Antineoplastics; fluorinated hydrocarbons, pyrimidines, small molecules |
Mechanism of action | Luteinizing hormone-releasing hormone (LHRH) receptor antagonist |
Route of administration | Oral |
Pharmacodynamics | Rapidly and dose-dependently suppresses luteinizing hormone (LH), follicle-stimulating hormone (FSH) and estradiol |
Pharmacokinetics | Dose-proportional pharmacokinetics; rapid absorption (time to peak plasma concentration of ≈ 1 h) and elimination (terminal elimination half-life of 4–6 h) |
Adverse reactions (≥ 5%) | Hot flushes and night sweats, headache, nausea, insomnia, amenorrhea, anxiety, arthralgia, depression-related adverse reactions, mood changes |
Serious adverse reactions | Bone density loss; changes in menstrual bleeding pattern and ability to recognise pregnancy; suicidal ideation, suicidal behaviour and exacerbation of mood disorders; hepatic transaminase elevations |
ATC codes | |
WHO ATC code | G03X-A (antigonadotrophins and similar agents); L02B (hormone antagonists and related agents) |
EphMRA ATC code | G3X (other sex hormones and similar products); L2B (cytostatic hormone antagonists) |
Chemical name | Butanoic acid, 4-[[(1R)-2-[5-(2-fluoro-3-methoxyphenyl)-3-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-3,6-dihydro-4-methyl-2,6-dioxo-1(2H)-pyrimidinyl]-1-phenylethyl]amino] |
2.3 Therapeutic Trials
2.3.1 Endometriosis
Elagolix 150 mg once daily and 200 mg twice daily improved dysmenorrhea symptoms and nonmenstrual pelvic pain in women with endometriosis and moderate to severe endometriosis-associated pain in the ELARIS EM-I and ELARIS EM-II trials [11]. Compared with placebo recipients, significantly greater proportions of elagolix 150 mg once daily and 200 mg twice daily recipients had clinical responses for dysmenorrhea (46.4 and 75.8% vs. 19.6%; p < 0.001 for both comparisons) and nonmenstrual pelvic pain (50.4 and 54.5% vs. 36.5%; p < 0.001 for both comparisons) at 3 months (primary endpoints) in ELARIS EM-I (Violet PETAL; NCT01620528) [11]. Clinical response rates for dysmenorrhea and nonmenstrual pelvic pain were sustained, remaining significantly higher with both elagolix doses versus placebo at 6 months (p ≤ 0.008 for all comparisons). Both elagolix doses were associated with significantly greater reductions in endometriosis-associated pain from baseline to month 3 than placebo (p < 0.001) and elagolix 200 mg twice daily (but not 150 mg once daily) reduced the use of rescue analgesics relative to placebo at both 3 months and 6 months (p < 0.001) [11]. Of the 872 women randomized in ELARIS EM-I [11], 149 elagolix 150 mg once daily recipients and 138 elagolix 200 mg twice daily recipients entered the double-blind ELARIS EM-III extension study (NCT01760954) [12]. After an additional 6 months of elagolix therapy at the same dose in ELARIS EM-III (i.e. after 12 continuous treatment months), elagolix 150 mg once daily and 200 mg twice daily recipients had response rates of 52.1 and 78.2%, respectively, for dysmenorrhea, 67.5 and 69.1% for nonmenstrual pelvic pain, and 45.2 and 60.0% for dyspareunia [12].
Clinical response rates were significantly higher in elagolix recipients than placebo recipients with respect to dysmenorrhea (43.4 and 72.4% vs. 22.7%; p < 0.001 for both comparisons) and nonmenstrual pelvic pain (49.8 and 57.8% vs. 36.5%; p = 0.003 and p < 0.001, respectively) at 3 months (primary endpoints) in ELARIS EM-II (Solstice; NCT01931670) [11]. These significant differences in clinical response rates were maintained at 6 months (p ≤ 0.01 for all comparisons). Both elagolix doses significantly improved change from baseline in endometriosis-associated pain at 3 months (p < 0.001 vs. placebo) and elagolix 200 mg twice daily (but not 150 mg once daily) reduced the use of rescue analgesics relative to placebo at both 3 months and 6 months (p < 0.001 vs. placebo) [11]. Of the 817 women randomized in ELARIS EM-II [11], 142 elagolix 150 mg once daily recipients and 140 elagolix 200 mg twice daily recipients proceeded to enter the double-blind ELARIS EM-IV extension study (NCT02143713) [12]. At 12 months (following an additional 6 months of elagolix therapy at the same dose in ELARIS EM-IV), elagolix 150 mg once daily and 200 mg twice daily recipients had response rates of 50.8 and 75.9% for dysmenorrhea, 66.4 and 67.2% for nonmenstrual pelvic pain, and 45.9 and 58.1% for dyspareunia [12].
ELARIS EM-I and EM-II were randomized, double-blind, multicentre, phase III trials that enrolled premenopausal women aged 18–49 years with a surgical diagnosis of endometriosis within the previous 10 years and moderate to severe endometriosis-associated pain [including a Composite Pelvic Signs and Symptoms Score (CPSSS) of ≥ 6 with scores of ≥ 2 for dysmenorrhea and nonmenstrual pelvic pain at screening] [11]. The phase II trials discussed below enrolled women aged 18–45 [13] or 18–49 years [14,15,16] with laparoscopically-confirmed endometriosis and a CPSSS of ≥ 6 (with scores of ≥ 2 for dysmenorrhea and ≥ 1 [13, 16] or ≥ 2 [14, 15] for nonmenstrual pelvic pain) [13,14,15,16].
Relative to placebo, elagolix was associated with significantly greater reductions in dysmenorrhea (p < 0.0001), non-menstrual pelvic pain (p = 0.0066) and dyspareunia scores (p = 0.007) from baseline to week 8 (primary outcomes) in the multicentre, phase II Daisy PETAL trial (NCT00973973) [15]. In this trial, 137 women were randomized to elagolix 150 mg once daily or placebo for an 8-week double-blind period and a subsequent 16-week open-label period [15].
Elagolix significantly improved reductions from baseline in monthly mean pelvic pain relative to placebo (p < 0.05 for elagolix 150 and 250 mg once daily vs. placebo at week 4 and elagolix 250 mg vs. placebo at week 8; no differences at week 12) in the randomized, double-blind, multinational, phase II Tulip PETAL trial (NCT00797225) [13]. In this 24-week trial, women (n = 174) received elagolix 150 mg once daily, elagolix 250 mg once daily, intramuscular monthly leuprorelin acetate or placebo; patients assigned to leuprorelin or placebo were re-randomized to elagolix at week 12 [13].
Compared with placebo, elagolix 150 and 250 mg once daily did not significantly improve reductions in monthly mean endometriosis-associated pain from baseline to week 12 (primary efficacy measure) in the randomized, double-blind, multicentre, phase II Lilac PETAL trial (NCT00619866) [16]. Participants (n = 155) received elagolix for 24 weeks or placebo for 12 weeks with re-randomization to an elagolix dose for the remaining 12 weeks [16].
Elagolix 150 mg once daily and 75 mg twice daily were associated with least-squares mean (LSM) changes in total CPSSS of −5.5 and −5.2 from baseline to week 24 in the randomized, double-blind, multicentre phase II PETAL trial (NCT00437658), while subcutaneous depot medroxyprogesterone acetate (injected at weeks 1 and 12) was associated with a LSM change of −5.3 [14]. Women in PETAL received 24 weeks of double-blind treatment (n = 84 per treatment arm) [14].
2.3.2 Uterine Leiomyoma
Elagolix 300 mg twice daily in combination with low-dose hormone add-back therapy reduced heavy menstrual bleeding. Significantly more elagolix than placebo recipients achieved a clinical response at month 6 (68.5 vs. 8.7%; p < 0.001) in the randomized, double-blind, multicentre phase III ELARIS UF-I trial (NCT02654054; M12-815) [17].
Similarly, elagolix 300 mg twice daily in combination with low-dose hormone add-back therapy reduced heavy menstrual bleeding in a significantly higher proportion of patients than placebo (76.2 vs. 10.1% achieving a clinical response at month 6; p < 0.001) in the randomized, double-blind, multicentre phase III ELARIS UF-II trial (NCT02691494; M12-817) [18].
Clinical responses with elagolix therapy were durable. Elagolix 300 mg twice daily in combination with low-dose hormone add-back therapy reduced heavy menstrual bleeding for up to 12 months in the randomized, double-blind, multicentre phase III ELARIS UF-EXTEND extension study (NCT02925494; M12-816), with 87.9% of women achieving a clinical response at month 12 [19]. In ELARIS UF-EXTEND, patients who had received elagolix 300 mg twice daily with or without hormone add-back therapy in ELARIS UF-I or –II received an additional 6 months of the same treatment, while patients who had initially received placebo were randomized to either of the two active treatment groups [19].
Significantly higher proportions of patients receiving elagolix monotherapy, elagolix in combination with low-dose hormone add-back therapy and elagolix in combination with standard-dose hormone add-back therapy achieved clinical responses than patients receiving placebo (92, 85 and 79% vs. 27% at month 6; all p < 0.001 vs. placebo) in the randomized, double-blind, multinational phase IIb (NCT01817530; M12-813) [cohort 1 results; n = 259] [20]. Mean endometrial thickness decreased by 0.52, 1.33 and 0.56 mm from baseline to month 6 in the respective elagolix groups, while increasing by 2.1 mm with placebo (cohort 1; p ≤ 0.05 for elagolix plus low-dose hormone therapy vs. placebo) [21]. Compared with the placebo group, elagolix groups had significantly greater mean reductions in symptom severity and improvements in health-related quality of life from baseline to month 6 (cohort 1; p ≤ 0.001 for all comparisons) [22]. Efficacy results in cohort 2 were consistent with those in cohort 1 [20,21,22]. In each cohort, patients were assigned to elagolix, elagolix in combination with low- or standard -dose hormone add-back therapy, or placebo [21]. Elagolix was administered at 300 mg twice daily in cohort 1 and 600 mg once daily in cohort 2 [21].
Elagolix significantly improved mean percentage change in MBL from baseline to month 3 relative to placebo (reductions of 72–98% with elagolix vs. reductions of 8–41% with placebo; p ≤ 0.01 for all doses vs. their respective placebo) in the randomized, dose-ranging, proof-of-concept phase IIa study (NCT01441635; M12-663) [23]. The highest mean percentage reduction was in women receiving elagolix 300 mg twice daily (98% vs. 41% with placebo; p ≤ 0.001). In the placebo-controlled treatment groups, patients received elagolix 100, 200 or 300 mg twice daily, or elagolix 400 mg once daily for 3 months (n = 33, 35, 30 and 32, respectively) [23].
The trials discussed in this section enrolled premenopausal women aged 18–51 years [17,18,19, 22] or 20–49 years [23] with heavy menstrual bleeding associated with uterine fibroids [17,18,19, 22, 23]. In the phase IIb trial [20]and the replicate, phase III ELARIS UF-I [17] and -II [18] trials, the primary endpoint was the percentage of responders based on menstrual blood loss (MBL) volume reduction at the final month. Clinical response was defined as MBL < 80 mL and ≥ 50% reduction from baseline, as measured using the alkaline hematin method [17,18,19,20].
Key clinical trials of elagolix, sponsored by AbbVie
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Elagolix ± estradiol/NETA | Moderate to severe endometriosis-associated pain | III | Recruiting | USA; Canada | NCT03343067; M16-383 |
Elagolix ± estradiol/NETA, placebo | Moderate to severe endometriosis-associated pain | III | Recruiting | USA; Canada; Puerto Rico | NCT03213457; M14-702 |
Elagolix, placebo | Moderate to severe endometriosis-associated pain | III | Completed | Multinational | NCT02143713; M12-821; 2013-001047-31 (ELARIS EM-IV) |
Elagolix, placebo | Moderate to severe endometriosis-associated pain | III | Completed | Multinational | NCT01931670; M12-671; 2011-004295-11 (ELARIS EM-II; Solstice) |
Elagolix, placebo | Moderate to severe endometriosis-associated pain | III | Completed | USA; Canada; Puerto Rico | NCT01760954; M12-667 (ELARIS EM-III) |
Elagolix, placebo | Moderate to severe endometriosis-associated pain | III | Completed | USA; Canada; Puerto Rico | NCT01620528; M12-665 (ELARIS EM-I; Violet PETAL) |
Elagolix + estradiol/NETA, placebo | Heavy menstrual bleeding associated with uterine fibroids | IIIb | Recruiting | USA; Puerto Rico | NCT03271489; M16-283 |
Elagolix ± estradiol/NETA | Heavy menstrual bleeding associated with uterine fibroids | III | Active, not recruiting | USA; Canada; Puerto Rico | NCT02925494; M12-816 (ELARIS UF-EXTEND) |
Elagolix ± estradiol/NETA, placebo | Heavy menstrual bleeding associated with uterine fibroids | III | Active, not recruiting | USA; Canada; Puerto Rico | NCT02654054; M12-815 (ELARIS UF-I) |
Elagolix ± estradiol/NETA, placebo | Heavy menstrual bleeding associated with uterine fibroids | III | Active, not recruiting | USA; Canada | NCT02691494; M12-817 (ELARIS UF-II) |
Elagolix, placebo | Endometriosis-associated pain | II | Completed | USA | NCT00973973; NBI-56418-0901 (Daisy PETAL) |
Elagolix, leuprorelin, placebo | Endometriosis-associated pain | II | Completed | Multinational | NCT00797225; NBI-56418-0703; 2007-006474-28 (Tulip PETAL) |
Elagolix, placebo | Endometriosis-associated pain | II | Completed | USA | NCT00619866; NBI-56418-0702 (Lilac PETAL) |
Elagolix, DMPA-SC | Endometriosis-associated pain | II | Completed | USA | NCT00437658; NBI-56418-0603 (PETAL) |
Elagolix, placebo | Endometriosis-associated pain | II | Completed | USA | NCT00109512; NBI-56418-0501 |
Elagolix ± estradiol/NETA, placebo | Heavy menstrual bleeding associated with uterine fibroids | IIb | Completed | Multinational | NCT01817530; M12-813; 2013-000082-37 |
Elagolix ± estradiol/NETA, estradiol or cyclical progesterone, placebo | Heavy menstrual bleeding associated with uterine fibroids | IIa | Completed | USA; Puerto Rico | NCT01441635; M12-663 |
2.4 Adverse Events
Elagolix was generally well tolerated in women with endometriosis [11,12,13,14,15,16] or uterine leiomyoma [23] in clinical trials, with most adverse events (AEs) being of mild to moderate severity.
In the ELARIS EM-I and –II trials in women with moderate to severe endometriosis-associated pain, AEs were reported in 79−81% of elagolix 150 mg once daily recipients and 83−85% of elagolix 200 mg twice daily recipients (vs. 72−74% of placebo recipients; p < 0.05 and p < 0.001 for the higher elagolix dose vs. placebo in ELARIS EM-I and –II, respectively) [11]. In a pooled analysis of the two trials, the most common treatment-emergent adverse reactions (incidence ≥ 5% of women in either elagolix dose group and higher than with placebo) were hot flush or night sweats (24 and 46% with elagolix 150 mg once daily and 200 mg twice daily vs. 9% with placebo), headache (17 and 20% vs. 12%), nausea (11 and 16% vs. 13%), insomnia (6 and 9% vs. 3%), altered mood/mood swings (6 and 5% vs. 3%), amenorrhea (4 and 7% vs. < 1%), depressed mood, depression, depressive symptoms and/or tearfulness (3 and 6% vs. 2%), anxiety (3 and 5% vs. 3%) and arthralgia (3 and 5% vs. 3%) [1]. Adverse reactions led to treatment discontinuation in 5.5% of elagolix 150 mg once daily recipients and 9.6% of elagolix 200 mg twice daily recipients (vs. 6.0% of placebo recipients) [1].
In ELARIS EM-I and –II, serious AEs occurred in 1−5% of elagolix 150 mg once daily recipients and 2−3% of elagolix 200 mg twice daily recipients (vs. 3% of placebo recipients) [11]. The most common serious adverse events in elagolix recipients included appendicitis (0.3%), abdominal pain (0.2%) and back pain (0.2%) [1].
Both elagolix groups had significantly greater mean decreases in bone mineral density (BMD) [lumbar spine, femoral neck and total hip] from baseline to 6 months than the placebo group (p < 0.05 for all comparisons in both trials, with the exception of femoral neck BMD with elagolix 150 mg once daily in ELARIS EM-I) [11]. During 6 months of treatment in ELARIS EM-I, lumbar spine, femoral neck or total hip BMD decreases > 8% (any time-point) were observed in 2% of elagolix 150 mg once daily recipients and 7% of elagolix 200 mg twice daily recipients (vs. < 1% of placebo recipients); during 6 months of treatment in ELARIS EM-II, the corresponding percentages were < 1 and 6% (vs. 0%) [1].
With respect to effects on menstrual bleeding patterns, the mean number of bleeding/spotting days in prior 28 days was 2.8 with elagolix 150 mg once daily, 0.8 with elagolix 200 mg twice daily and 4.6 with placebo after 3 months of treatment in ELARIS EM-I or –II [1]. During 6 months of treatment, the incidence of amenorrhea (no bleeding or spotting in a 56-day interval) was 6–17% in elagolix 150 mg once daily recipients, 13–52% in elagolix 200 mg twice daily recipients and < 1% in placebo recipients [1].
Suicidal ideation was reported in 0.2% of patients receiving either elagolix dose in the pivotal trials and was not reported in any placebo recipients [1]. There was one patient death; suicide by overdose with multiple non-trial drugs in an elagolix 150 mg once daily recipient [1, 11].
In the pivotal trials, asymptomatic alanine aminotransferase (ALT) elevations to ≥ 3 × the upper limit of normal of the reference range occurred in 0.2% of elagolix 150 mg once daily recipients, 1.1% of elagolix 200 mg twice daily recipients and 0.1% of placebo recipients [1]. An increase in LDL cholesterol to ≥ 190 mg/dL occurred in 12% of elagolix recipients and 1% of placebo recipients with mildly elevated LDL cholesterol (130–159 mg/dL) at baseline, while an increase in serum triglycerides to ≥ 500 mg/dL occurred in 4% of elagolix recipients and 1% of placebo recipients with a mild elevation (150–300 mg/dL) at baseline [1].
Longer-term data from the ELARIS EM-III and –IV extension trials revealed no new safety signals [12]. During the extension trials, decreased BMD was the most common cause of treatment discontinuation (0.3 and 3.6% of elagolix 150 mg once daily and 200 mg twice daily recipients) [1].
AEs with elagolix (with or without hormone add-back therapy) in women with heavy menstrual bleeding associated with uterine fibroids participating in the phase IIa M12-663 trial were broadly consistent with those seen in women with endometriosis [23]. Hot flush was the most common treatment-emergent AE, occurring in 46–63% of elagolix monotherapy recipients, 19–27% of elagolix plus hormone add-back therapy recipients and 12% of placebo recipients [23].
2.5 Ongoing Clinical Trials
Elagolix with or without hormone add-back therapy (estradiol/norethindrone acetate) is being evaluated in ongoing clinical trials sponsored by AbbVie.
Recruitment is currently underway for two randomized, phase III trials that will evaluate elagolix as both monotherapy and in combination with hormone add-back therapy in the management of moderate to severe endometriosis-associated pain in premenopausal women. The M16-383 dose-escalation trial (NCT03343067) is enrolling patients in the USA and Canada, while the placebo-controlled M14-702 trial (NCT03213457) is enrolling in the USA, Canada and Puerto Rico.
A randomized, placebo-controlled phase IIIb trial (NCT03271489; M16-283) of elagolix with or without estradiol/norethindrone acetate in the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women is currently recruiting participants in the USA and Puerto Rico. Phase III trials currently evaluating elagolix as both monotherapy and in combination with hormone add-back therapy in the same indication include the ELARIS UF-I trial, underway at centres in the USA, Canada and Puerto Rico, and the ELARIS UF-II trial in the USA and Canada; women who have been treated in ELARIS UF-I or –II have the option of entering the ongoing extension study (ELARIS UF-EXTEND).
3 Current Status
Elagolix received its first global approval on 23 July 2018 in the USA for the management of moderate to severe pain associated with endometriosis.
Change history
15 November 2018
The original article can be found online.
References
AbbVie Inc. Prescribing information for OrilissaTM (elagolix) tablets, for oral use. 2018. http://www.accessdata.fda.gov/. Accessed 15 Aug 2018.
Ng J, Chwalisz K, Carter DC, et al. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J Clin Endocrinol Metab. 2017;102(5):1683–91.
Struthers RS, Nicholls AJ, Grundy J, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab. 2009;94(2):545–51.
AbbVie. AbbVie receives US FDA approval of ORILISSA™ (elagolix) for the management of moderate to severe pain associated with endometriosis. 2018. https://news.abbvie.com/. Accessed 15 Aug 2018.
Abbott. Abbott and Neurocrine announce global agreement to develop and commercialize elagolix for the treatment of endometriosis [media release] 16 Jun 2010. http://www.abbott.com
Neurocrine Biosciences Inc. Neurocrine biosciences reports third quarter 2010 results [media release] 28 Oct 2010. http://www.neurocrine.com
Neurocrine Biosciences Inc. Form 10-K. 2015. https://www.sec.gov/. Accessed 7 Aug 2018.
Winzenborg I, Nader A, Polepally AR, et al. Population pharmacokinetics of elagolix in healthy women and women with endometriosis. Clin Pharmacokinet. 2018. https://doi.org/10.1007/s40262-018-0629-6.
Ng J, Salem A, Carter D, et al. Effects of the coadministration of single and multiple doses of rifampin on the pharmacokinetics and safety of elagolix in healthy premenopausal females. Clin Pharmacol Ther. 2016;99(Suppl 1):S54–5.
Ng J, Salem A, Carter D, et al. Effect of the coadministration of ketoconazole on the pharmacokinetics and safety of elagolix in healthy premenopausal females. Clin Pharmacol Ther. 2016;99(Suppl 1):S55.
Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–60.
Acs N, O’Brien C, Jiang P, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist: results from a phase 2, randomized controlled study. J Endometr Pelvic Pain Disord. 2015;7(2):56–62.
Carr B, Dmowski WP, O’Brien C, et al. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: effects on bone mineral density. Reprod Sci. 2014;21(11):1341–51.
Carr B, Giudice L, Dmowski WP, et al. Elagolix, an oral GnRH antagonist for endometriosis-associated pain: a randomized controlled study. J Endometr Pelvic Pain Disord. 2013;5(3):105–15.
Diamond MP, Carr B, Dmowski WP, et al. Elagolix treatment for endometriosis-associated pain: results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod Sci. 2014;21(3):363–71.
AbbVie, Neurocrine Biosciences Inc. AbbVie announces positive topline results from phase 3 study evaluating investigational elagolix in women with uterine fibroids [media release]. http://www.abbvie.com. Accessed 21 Feb 2018.
AbbVie. AbbVie announces positive topline results from second phase 3 study evaluating investigational elagolix in women with uterine fibroids [media release]. http://www.abbvie.com. Accessed 13 Mar 2018.
AbbVie. AbbVie announces positive topline results from phase 3 extension study evaluating investigational elagolix in women with uterine fibroids [media release]. http://www.abbvie.com. Accessed 22 Aug 2018.
Stewart E, Owens C, Duan WR, et al. Elagolix alone and with add-back decreases heavy menstrual bleeding in women with uterine fibroids. Conference Abstract. 2017.
Simon JA, Stewart EA, Owens C, et al. Elagolix treatment in women with heavy menstrual bleeding-associated with uterine fibroids: Efficacy and safety results from a phase 2b study. Fertil Steril. 2017;108(3 Suppl):e26–7.
Diamond M, Soliman AM, Gao J, et al. Elagolix improves quality of life in women with heavy menstrual bleeding associated with uterine fibroids: evidence from a phase 2b randomized trial. Fertil Steril. 2017;108(3 Suppl):e27.
Archer DF, Stewart EA, Jain RI, et al. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertil Steril. 2017;108(1):152–60.e4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The original version of this article was revised due to a retrospective Open Access request.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Lamb, Y.N. Elagolix: First Global Approval. Drugs 78, 1501–1508 (2018). https://doi.org/10.1007/s40265-018-0977-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0977-4