Abstract
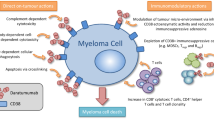
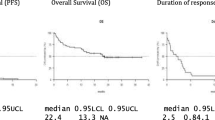
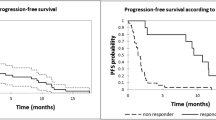
Intravenous daratumumab (DARZALEX®) is a first-in-class human IgG1κ monoclonal antibody against CD38 available for use in patients with relapsed and/or refractory multiple myeloma. In phase I/II and II trials and a pooled analysis of these studies, daratumumab monotherapy induced an overall response (partial response or better) in approximately one-third of patients; responses were rapid, deep and durable. An overall survival (OS) benefit was seen with daratumumab monotherapy, including in patients with a minimal response or stable disease. In phase III trials, daratumumab in combination with either bortezomib plus dexamethasone or lenalidomide plus dexamethasone significantly prolonged progression-free survival and induced deep and durable responses compared with bortezomib plus dexamethasone or lenalidomide plus dexamethasone. An OS benefit with daratumumab triple combination therapy is yet to be demonstrated (as the OS data were not mature at the time of the last analysis). Daratumumab was generally well tolerated when used as monotherapy and had a generally manageable tolerability profile when used in combination therapy. Infusion-related reactions (IRRs) were the most common adverse events; these were predominantly grade 1 or 2 and mostly occurred during the first infusion. The most common grade 3–4 adverse events associated with daratumumab triple combination therapy were thrombocytopenia, neutropenia and anaemia. Although final OS data are awaited, current evidence indicates that daratumumab is a valuable addition to the treatment options currently available for patients with relapsed or refractory multiple myeloma.

Similar content being viewed by others
Change history
24 January 2018
The author has alerted us to the following error in Sect. 4.2.2.1, and the following correction should be noted:
References
Kumar SK, Callander NS, Alsina M, et al. Multiple myeloma, version 3.2017: NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(2):230–69.
Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–57.
Usmani S, Ahmadi T, Ng Y, et al. Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;02(21):1355–61.
Lonial S, Durie B, Palumbo A, et al. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia. 2016;30(3):526–35.
Janssen Biotech Inc. Darzalex (daratumumab) injection, for intravenous use: US prescribing information. 2016. http://www.janssenmd.com. Accessed 25 Oct 2017.
Genmab. Genmab announces approval of DARZALEX® (daratumumab) for relapsed or refractory multiple myeloma in Japan [media release]. 2017. http://ir.genmab.com.
European Medicines Agency. Darzalex, concentrate for solution for infusion: EU summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 25 Oct 2017.
de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8.
Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197(3):807–13.
Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7(2):311–21.
Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94.
Nijhof IS, Groen RWJ, Noort WA, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21(12):2802–10.
van der Veer MS, de Weers M, van Kessel B, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011;96(2):284–90.
Nijhof IS, Casneuf T, van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–70.
Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion (Paris). 2015;55(6 Pt 2):1545–54.
Clemens PL, Yan X, Lokhorst HM, et al. Pharmacokinetics of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitor and immunomodulatory drug treatment. Clin Pharmacokinet. 2016;56(8):915–24.
Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–19.
Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–60.
Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37–44.
Salomon-Perzynski A, Walter-Croneck A, Usnarska-Zubkiewicz L, et al. Real-world results of daratumumab monotherapy in heavily pretreated relapsed/refractory multiple myeloma in Poland: a prospective observational study of the Polish myeloma group [abstract no. E1262]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Plesner T, Arkenau H-T, Gimsing P, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016;128(14):1821–8.
Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–66.
Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–31.
Chanan-Khan AA, Lentzsch S, Quach H, et al. Daratumumab, bortezomib and dexamethasone versus bortezomib and dexamethasone alone for relapsed or refractory multiple myeloma based on prior treatment exposure: updated efficacy analysis of Castor [abstract no. 3313]. Blood. 2016;128(22).
Lentzsch S, Nooka A, Quach H, et al. Daratumumab, bortezomib and dexamethasone (DVd) vs bortezomib and dexamethasone (Vd) in RRMM based on prior lines and treatment exposure: CASTOR [abstract no. PS-238 (d)]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
Mateos M-V, Estell J, Barreto W, et al. Efficacy of daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory myeloma based on prior lines of therapy: updated analysis of Castor [abstract no. 1150]. Blood. 2016;128(22).
Mateos M-V, Spencer A, Nooka A, et al. Daratumumab-based combination regimens in elderly (≥75 years) patients with relapsed or refractory multiple myeloma (RRMM): subgroup analysis of the phase 3 CASTOR and POLLUX studies [abstract no. P335 plus poster]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
San-Miguel J, Weisel K, Cook G, et al. Efficacy by cytogenetic risk status for daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone in relapsed or refractory multiple myeloma [abstract no. S101]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Spencer A, Mark T, Spicka I, et al. Depth of response and MRD with daratumumab plus bortezomib and dexamethasone (DVd) vs bortezomib and dexamethasone (Vd) in RRMM: CASTOR [abstract no. PS-151 (d)]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
Lentzsch S, Weisel K, Mateos M-V, et al. Daratumumab, bortezomib and dexamethasone (DVd) vs bortezomib and dexamethasone (Vd) in relapsed or refractory multiple myeloma (RRMM): efficacy and safety update (CASTOR) [abstract no. 8036 plus poster]. In: Annual meeting of the American Society of Clinical Oncology (ASCO); 2017.
Usmani SZ, Dimopoulos MA, Belch A, et al. Efficacy of daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma patients with 1 to 3 prior lines of therapy: updated analysis of Pollux [abstract no. 1151]. Blood. 2016;128(22).
Moreau P, Kaufman JL, Sutherland HJ, et al. Efficacy of daratumumab, lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone for relapsed or refractory multiple myeloma among patients with 1 to 3 prior lines of therapy based on previous treatment exposure: updated analysis of Pollux [abstract no. 489]. Blood. 2016;128(22).
Dimopoulos MA, Belch AR, White DJ, et al. Daratumumab, lenalidomide and dexamethasone (DRd) vs lenalidomide and dexamethasone (Rd) in RRMM based on prior lines and treatment exposure: POLLUX [abstract no. PS-207 (d)]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
San-Miguel J, Dimopoulos MA, Usmani S, et al. Depth of response and MRD with daratumumab plus lenalidomide and dexamethasone (DRd) vs lenalidomide and dexamethasone (Rd) in RRMM: POLLUX [abstract no. OP-028]. In: 16th international myeloma workshop, Delhi, India, 1–4 Mar 2017.
Dimopoulos M, Moreau P, Nahi H, et al. Efficacy and safety of daratumumab, lenalidomide, and dexamethasone (DRd) versus Rd alone in relapsed or refractory multiple myeloma (RRMM): updated analysis of POLLUX [abstract no. P334 plus poster]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):52–61.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): multiple myeloma (version 2.2018). 2017. http://www.nccn.org/. Accessed 26 Oct 2017.
Harousseau JL, Attal M. How I treat first relapse of myeloma. Blood. 2017;130(8):963–73.
Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–46.
Anderson KC, Auclair D, Kelloff GJ, et al. The role of minimal residual disease testing in myeloma treatment selection and drug development: current value and future applications. Clin Cancer Res. 2017;23(15):3980–93.
Anderson KC. Should minimal residual disease negativity be the end point of myeloma therapy? Blood Adv. 2017;1(8):517–21.
Sonneveld P. Should minimal residual disease negativity not be the end point of myeloma therapy? Blood Adv. 2017;1(8):522–5.
Usmani SZ, Diels J, Ito T, et al. Daratumumab monotherapy compared with historical control data in heavily pretreated and highly refractory patients with multiple myeloma: an adjusted treatment comparison. Am J Hematol. 2016;92:E146–52.
Hájek R, Jelinek T, Maisnar V, et al. Comparative effectiveness of daratumumab monotherapy versus a real-world historical control from the Czech Republic in heavily pretreated and highly refractory multiple myeloma patients [abstract no. 3332]. Blood. 2016;128(22).
Kumar SK, Durie BGM, Su Z, et al. Adjusted comparisons suggest daratumumab is associated with prolonged survival compared with standard of care therapies in patients with heavily pre-treated and highly refractory multiple myeloma [abstract no. 4517]. Blood. 2016;128(22).
Botta C, Ciliberto D, Rossi M, et al. Network meta-analysis of randomized trials in multiple myeloma: efficacy and safety in relapsed/refractory patients. Blood Adv. 2017;1(7):455–66.
van Beurden-Tan CHY, Franken MG, Blommestein HM, et al. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35(12):1312–9.
Dimopoulos M, Weisel K, Kaufman JL, et al. Efficacy of daratumumab-based regimens in patients with relapsed/refractory multiple myeloma—a systematic literature review and network meta-analysis [abstract no. E1281 plus poster]. In: 22nd Congress of the European Hematology Association (EHA); 2017.
Rajkumar SV, Harousseau JL. Next-generation multiple myeloma treatment: a pharmacoeconomic perspective. Blood. 2016;128(24):2757–64.
National Institute for Health and Care Excellence. Appraisal consultation document: daratumumab monotherapy for treating relapsed and refractory multiple myeloma. 2017. http://www.nice.org.uk. Accessed 25 Oct 2017.
Alsaid N, McBride A, Agarwal AB, et al. Cost effectiveness of carfilzomib (CAR), ixazomib (IXA), elotuzumab (ELO), or daratumumab (DAR) with lenalidomide and dexamethasone (LEN+DEX) vs LEN+DEX in relapsed/refractory multiple myeloma (R/R MM) [abstract no. 8030]. In: Annual meeting of the American Society of Clinical Oncology (ASCO); 2017.
Acknowledgements
During the peer review process, the manufacturer of daratumumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Hannah Blair is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/3AF8F06079DC09B5.
Additional information
The manuscript was reviewed by: S. Kumar, Hematology and Internal Medicine, Mayo Clinic, Rochester, MN, USA; H. Lokhorst, Department of Hematology, VU University Medical Center, Amsterdam, Netherlands; T. M. Mark, University of Colorado Cancer Center, Division of Hematology, Aurora, CO, USA; A. Oriol, Hospital Universitario Germans Trias I Pujol, Badalona, Spain.
Rights and permissions
About this article
Cite this article
Blair, H.A. Daratumumab: A Review in Relapsed and/or Refractory Multiple Myeloma. Drugs 77, 2013–2024 (2017). https://doi.org/10.1007/s40265-017-0837-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0837-7