Abstract
Background
Both chronic and acute pain have been cited as the most common symptoms amongst patients with multiple sclerosis (MS), with recent prevalence estimates as high as 83 %. The evidence for spasticity and trigeminal neuralgia pharmacological treatments in MS has been systematically reviewed, but no equivalent reviews have been published concerning MS pain unrelated to these two conditions.
Objective
Our objective was to systematically review pain management strategies for the reduction of non-spastic and non-trigeminal neuralgic pain in MS patients.
Data Sources
Experimental studies published after 1965 were chosen for review by searching electronic databases (e.g. PubMed, Cumulative Index to Nursing and Allied Health Literature, Science Citation Index Expanded, Conference Proceedings Citation Index-Science, and clinicaltrials.gov) and bibliographies/citations of previously published reviews.
Study Selection
Studies were included if all participants were adults clinically diagnosed with MS, study sample was not restricted to participants with spasticity or trigeminal neuralgia, and participant-reported pain was a primary or secondary outcome measured with a validated tool.
Study Appraisal and Synthesis Methods
Records were screened and methodological qualities of included studies were assessed independently by two reviewers under the supervision of another reviewer using the principles recommended in the Cochrane Handbook for Systematic Review of Interventions and the levels of evidence espoused by the American Academy of Neurology.
Results
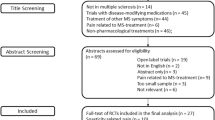
Fifteen studies met the inclusion and exclusion criteria for review; interventions included antidepressants, anticonvulsants, dextromethorphan/quinidine, cannabinoids, and opioids/opioid antagonists. The pooled effect size for anticonvulsants (4 studies, 78 participants) was −1.88 (95 % CI: −3.13 to −0.64). The pooled effect size for cannabinoids (3 studies, 565 participants) was 0.08 (95 % CI: −0.74 to 0.89). Overall, only four trials reported Class 1 evidence. For these trials, dizziness was the most commonly reported adverse event, followed by nausea and somnolence.
Limitations
The relatively small number of trials in MS patients with chronic pain precludes specific recommendations for treatment strategies. The review did not reveal any studies of drug combinations.
Conclusions
More trials with rigorous design and reporting are needed to determine effective treatments for specific pain types presenting in people living with MS.

Similar content being viewed by others
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–31.
Brichetto G, Messmer Uccelli M, Mancardi GL, Solaro C. Symptomatic medication use in multiple sclerosis. Mult Scler. 2003;9:458–60.
Aring CD. Pain in multiple sclerosis. JAMA. 1973;223:547.
O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137:96–111.
Solaro C, Uccelli MM. Management of pain in multiple sclerosis: a pharmacological approach. Nat Rev Neurol. 2011;7:519–27.
Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. 2011;12:2355–68.
Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e88.
Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–9.
Paisley S, Beard S, Hunn A, Wight J. Clinical effectiveness of oral treatments for spasticity in multiple sclerosis: a systematic review. Mult Scler. 2002;8:319–29.
De Santi L, Annunziata P. Symptomatic cranial neuralgias in multiple sclerosis: clinical features and treatment. Clin Neurol Neurosurg. 2012;114:101–7.
Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev. 2003;(4):CD001332.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Frontiers Neurosci. 2012;6:10.
Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6.
McDonald W, Halliday A. Diagnosis and classification of multiple sclerosis. Br Med Bull. 1977;33:4–8.
Poser C, Brinar VV. Diagnostic criteria for multiple sclerosis. Clin Neurol Neurosurg. 2001;103:1–11.
Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–31.
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–99.
Ware JE Jr. Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I: conceptual framework and item selection. Med Care. 1992;30:473–83.
Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–81.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–52.
Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605.
Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25.
Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18.
Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; 2011; No. Version 5.1.0 (updated March 2011).
French J, Gronseth G. Lost in a jungle of evidence: we need a compass. Neurology. 2008;71:1634–8.
Fishman B, Pasternak S, Wallenstein SL, Houde RW, Holland JC, Foley KM. The Memorial Pain Assessment Card: a valid instrument for the evaluation of cancer pain. Cancer. 1987;60:1151–8.
Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–18.
Falah M, Madsen C, Holbech JV, Sindrup SH. A randomized, placebo-controlled trial of levetiracetam in central pain in multiple sclerosis. Eur J Pain. 2012;16:860–9.
Rossi S, Mataluni G, Codeca C, et al. Effects of levetiracetam on chronic pain in multiple sclerosis: results of a pilot, randomized, placebo-controlled study. Eur J Neurol. 2009;16:360–6.
Breuer B, Pappagallo M, Knotkova H, Guleyupoglu N, Wallenstein S, Portenoy RK. A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clin Ther. 2007;29:2022–30.
Houtchens MK, Richert JR, Sami A, Rose JW. Open label gabapentin treatment for pain in multiple sclerosis. Mult Scler. 1997;3:250–3.
Solaro C, Boehmker M, Tanganelli P. Pregabalin for treating paroxysmal painful symptoms in multiple sclerosis: a pilot study. J Neurol. 2009;256:1773–4.
Solaro C, Restivo D, Mancardi GL, Tanganelli P. Oxcarbazepine for treating paroxysmal painful symptoms in multiple sclerosis: a pilot study. Neurol Sci. 2007;28:156–8.
Chitsaz A, Janghorbani M, Shaygannejad V, Ashtari F, Heshmatipour M, Freeman J. Sensory complaints of the upper extremities in multiple sclerosis: relative efficacy of nortriptyline and transcutaneous electrical nerve stimulation. Clin J Pain. 2009;25:281–5.
Eli Lilly & Co. Duloxetine for multiple sclerosis pain [ClinicalTrials.gov identifier NCT00755807]. US National Institutes of Health, ClinicalTrials.gov. Available from URL: http://www.clinicaltrials.gov. Accessed 28 Aug 2013.
GW Pharmaceuticals Ltd. Sativex versus placebo when added to existing treatment for central neuropathic pain in MS [ClinicalTrials.gov identifier NCT00391079]. US National Institutes of Health, ClinicalTrials.gov. Available from URL: http://www.clinicaltrials.gov. Accessed 28 Aug 2013.
Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–41.
Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329:253.
Panitch HS, Thisted RA, Smith RA, et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006;59:780–7.
Cree BA, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol. 2010;68:145–50.
Kalman S, Osterberg A, Sorensen J, Boivie J, Bertler A. Morphine responsiveness in a group of well-defined multiple sclerosis patients: a study with i.v. morphine. Eur J Pain. 2002;6:69–80.
Lakhan SE, Rowland M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009;9:59.
Olney N, Rosen H. AVP-923, a combination of dextromethorphan hydrobromide and quinidine sulfate for the treatment of pseudobulbar affect and neuropathic pain. IDrugs. 2010;13:254–65.
Pioro EP, Brooks BR, Cummings J, et al. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68:693–702.
Hobson K. Lawmakers aren’t laughing about Avanir’s price for nuedexta. Wall Street J. 2011. Available from: http://blogs.wsj.com/health/2011/05/25/lawmakers-arent-laughing-about-avanirs-price-for-nuedexta/. Accessed 6 March 2013.
Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81:1372–3.
Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;(3):CD007938.
Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076.
Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev. 2009;(4):CD007115.
Eisenberg E, McNicol E, Carr DB. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2006;(3):CD006146.
O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–32.
Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943.
Acknowledgments
Dr. Lapane serves as a consultant to Janssen. Dr. Oh, Dr. Jawahar and Mr. Yang have no conflicts of interest to report.
Study funding
No funds were received to support this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jawahar, R., Oh, U., Yang, S. et al. A Systematic Review of Pharmacological Pain Management in Multiple Sclerosis. Drugs 73, 1711–1722 (2013). https://doi.org/10.1007/s40265-013-0125-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0125-0