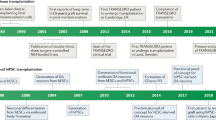
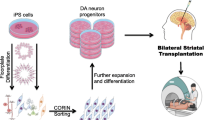
Abstract
There is escalating interest in cell-based therapies to restore lost dopamine inputs in Parkinson’s disease. This is based upon the rationale that implanting dopamine progenitors into the striatum can potentially improve dopamine-responsive motor symptoms. A rich body of data describing clinical trials of previous cell transplantation exists. These have included multiple cell sources for transplantation including allogeneic (human embryonic mesencephalic tissue, retinal pigment epithelial cells) and autologous (carotid body, adrenal medullary tissue) cells, as well as xenotransplantation. However, there are multiple limitations related to these cell sources, including availability of adequate numbers of cells for transplant, heterogeneity within cells transplanted, imprecisely defined mechanisms of action, and poor cell survival after transplantation in some cases. Nonetheless, evidence has accrued from a subset of trials to support the rationale for such a regenerative approach. Recent rapid advances in stem cell technology may now overcome these prior limitations. For example, dopamine neuron precursor cells for transplant can be generated from induced pluripotent cells and human embryonic stem cells. The benefits of these innovative approaches include: the possibility of scalability; a high degree of quality control; and improved understanding of mechanisms of action with rigorous preclinical testing. In this review, we focus on the potential for cell-based therapies in Parkinson’s disease to restore the function of dopaminergic neurons, we critically review previous attempts to harness such strategies, we discuss potential benefits and predicted limitations, and we address how previous roadblocks may be overcome to bring a cell-based approach to the clinic.

Similar content being viewed by others
References
Barker RA, Parmar M, Studer L, Takahashi J. Human trials of stem cell-derived dopamine neurons for Parkinson’s disease: dawn of a new era. Cell Stem Cell. 2017;21(5):569–73. https://doi.org/10.1016/j.stem.2017.09.014.
Henchcliffe C, Parmar M. Repairing the brain: cell replacement using stem cell-based technologies. J Parkinsons Dis. 2018;8(s1):S131–7. https://doi.org/10.3233/JPD-181488.
Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):11–2. https://doi.org/10.1016/j.cger.2019.08.002.
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42. https://doi.org/10.1111/ene.14108.
Zesiewicz TA. Parkinson disease. Continuum (Minneap Minn). 2019;25(4):896–918. https://doi.org/10.1212/CON.0000000000000764.
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and Movement Disorder Society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–66. https://doi.org/10.1002/mds.27372.
Fishman P, Lipsman N. Focused ultrasound as an evolving therapy for Parkinson’s disease. Mov Disord. 2019;34(9):1241–2. https://doi.org/10.1002/mds.27809.
Hitti FL, Yang AI, Gonzalez-Alegre P, Baltuch GH. Human gene therapy approaches for the treatment of Parkinson’s disease: an overview of current and completed clinical trials. Parkinsonism Relat Disord. 2019;66:16–24. https://doi.org/10.1016/j.parkreldis.2019.07.018.
Marques CR, Marote A, Mendes-Pinheiro B, Teixeira FG, Salgado AJ. Cell secretome based approaches in Parkinson’s disease regenerative medicine. Expert Opin Biol Ther. 2018;18(12):1235–45. https://doi.org/10.1080/14712598.2018.1546840.
Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12(1):84–91. https://doi.org/10.1016/S1474-4422(12)70295-8.
Parmar M. Towards stem cell based therapies for Parkinson’s disease. Development. 2018;145(1):ev.156117. https://doi.org/10.1242/dev.156117.
Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol. 2015;33(2):204–9. https://doi.org/10.1038/nbt.3124.
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–51. https://doi.org/10.1038/nature10648.
Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16(3):269–74. https://doi.org/10.1016/j.stem.2015.01.018.
Adler AF, Cardoso T, Nolbrant S, Mattsson B, Hoban DB, Jarl U, et al. hESC-derived dopaminergic transplants integrate into basal ganglia circuitry in a preclinical model of Parkinson’s disease. Cell Rep. 2019;28(13):3462–73.e5. https://doi.org/10.1016/j.celrep.2019.08.058.
Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–6. https://doi.org/10.1212/WNL.0b013e3181c1ded6.
Bohnen NI, Kanel P, Muller M. Molecular imaging of the cholinergic system in Parkinson’s disease. Int Rev Neurobiol. 2018;141:211–50. https://doi.org/10.1016/bs.irn.2018.07.027.
Brundin P, Barbin G, Strecker RE, Isacson O, Prochiantz A, Bjorklund A. Survival and function of dissociated rat dopamine neurones grafted at different developmental stages or after being cultured in vitro. Brain Res. 1988;467(2):233–43. https://doi.org/10.1016/0165-3806(88)90027-2.
Brundin P, Isacson O, Gage FH, Prochiantz A, Bjorklund A. The rotating 6-hydroxydopamine-lesioned mouse as a model for assessing functional effects of neuronal grafting. Brain Res. 1986;366(1–2):346–9. https://doi.org/10.1016/0006-8993(86)91316-8.
Brundin P, Nilsson OG, Strecker RE, Lindvall O, Astedt B, Bjorklund A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Exp Brain Res. 1986;65(1):235–40. https://doi.org/10.1007/bf00243848.
Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204(4393):643–7. https://doi.org/10.1126/science.571147.
Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177(3):555–60. https://doi.org/10.1016/0006-8993(79)90472-4.
Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247(4942):574–7.
Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease: a detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989;46(6):615–31.
Stromberg I, Herrera-Marschitz M, Hultgren L, Ungerstedt U, Olson L. Adrenal medullary implants in the dopamine-denervated rat striatum. I. Acute catecholamine levels in grafts and host caudate as determined by HPLC-electrochemistry and fluorescence histochemical image analysis. Brain Res. 1984;297(1):41–51. https://doi.org/10.1016/0006-8993(84)90541-9.
Herrera-Marschitz M, Stromberg I, Olsson D, Ungerstedt U, Olson L. Adrenal medullary implants in the dopamine-denervated rat striatum. II. Acute behavior as a function of graft amount and location and its modulation by neuroleptics. Brain Res. 1984;297(1):53–61. https://doi.org/10.1016/0006-8993(84)90542-0.
Morihisa JM, Nakamura RK, Freed WJ, Mishkin M, Wyatt RJ. Adrenal medulla grafts survive and exhibit catecholamine-specific fluorescence in the primate brain. Exp Neurol. 1984;84(3):643–53. https://doi.org/10.1016/0014-4886(84)90211-5.
Goetz CG, Olanow CW, Koller WC, Penn RD, Cahill D, Morantz R, et al. Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson’s disease. N Engl J Med. 1989;320(6):337–41. https://doi.org/10.1056/NEJM198902093200601.
Madrazo I, Drucker-Colin R, Diaz V, Martinez-Mata J, Torres C, Becerril JJ. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. N Engl J Med. 1987;316(14):831–4. https://doi.org/10.1056/NEJM198704023161402.
Lindvall O, Widner H, Rehncrona S, Brundin P, Odin P, Gustavii B, et al. Transplantation of fetal dopamine neurons in Parkinson’s disease: one-year clinical and neurophysiological observations in two patients with putaminal implants. Ann Neurol. 1992;31(2):155–65. https://doi.org/10.1002/ana.410310206.
Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Wells TH, Barrett JN, et al. Transplantation of human fetal dopamine cells for Parkinson’s disease: results at 1 year. Arch Neurol. 1990;47(5):505–12. https://doi.org/10.1001/archneur.1990.00530050021007.
Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327(22):1549–55. https://doi.org/10.1056/NEJM199211263272202.
Molina H, Quinones R, Ortega I, Alvarez L, Munoz J, Gonzalez C, et al. Computer assisted CT-guided stereotactic transplantation of foetal ventral mesencephalon to the caudate nucleus and putamen in Parkinson’s disease. Acta Neurochir Suppl (Wien). 1993;58:17–9.
Spencer DD, Robbins RJ, Naftolin F, Marek KL, Vollmer T, Leranth C, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N Engl J Med. 1992;327(22):1541–8. https://doi.org/10.1056/NEJM199211263272201.
Peschanski M, Defer G, N’Guyen JP, Ricolfi F, Monfort JC, Remy P, et al. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain. 1994;117(Pt 3):487–99.
Mendez I, Dagher A, Hong M, Gaudet P, Weerasinghe S, McAlister V, et al. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. J Neurosurg. 2002;96(3):589–96. https://doi.org/10.3171/jns.2002.96.3.0589.
Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol. 2015;11(9):492–503. https://doi.org/10.1038/nrneurol.2015.123.
Wenning GK, Odin P, Morrish P, Rehncrona S, Widner H, Brundin P, et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann Neurol. 1997;42(1):95–107. https://doi.org/10.1002/ana.410420115.
Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, et al. Sequential bilateral transplantation in Parkinson’s disease: effects of the second graft. Brain. 1999;122(Pt 6):1121–32.
Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain. 2000;123(Pt 7):1380–90.
Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71(1):83–7. https://doi.org/10.1001/jamaneurol.2013.4749.
Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep. 2014;7(6):1755–61. https://doi.org/10.1016/j.celrep.2014.05.027.
Henchcliffe C, Carter J, Hanineva A, Kang Y, Babich J, Gollomp SM, et al. Clinical and neuroimaging outcomes up to 18 years after fetal tissue transplant for Parkinson’s disease [abstract]. Mov Disord. 2016;31(Suppl. 2).
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–9. https://doi.org/10.1056/NEJM200103083441002.
Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–14. https://doi.org/10.1002/ana.10720.
Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, et al. Dopamine cell implantation in Parkinson’s disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med. 2010;51(1):7–15. https://doi.org/10.2967/jnumed.109.066811.
Martinez-Martin P, Jeukens-Visser M, Lyons KE, Rodriguez-Blazquez C, Selai C, Siderowf A, et al. Health-related quality-of-life scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2011;26(13):2371–80. https://doi.org/10.1002/mds.23834.
Titova N, Martinez-Martin P, Katunina E, Chaudhuri KR. Advanced Parkinson’s or “complex phase” Parkinson’s disease? Re-evaluation is needed. J Neural Transm (Vienna). 2017;124(12):1529–37. https://doi.org/10.1007/s00702-017-1799-3.
Lane EL, Winkler C. L-DOPA- and graft-induced dyskinesia following transplantation. Prog Brain Res. 2012;200:143–68. https://doi.org/10.1016/B978-0-444-59575-1.00007-7.
Barker RA, TRANSEURO Consortium. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat Med. 2019;25(7):1045–53. https://doi.org/10.1038/s41591-019-0507-2.
Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med. 2010;2(38):38ra46. https://doi.org/10.1126/scitranslmed.3000976.
Backlund EO, Granberg PO, Hamberger B, Knutsson E, Martensson A, Sedvall G, et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism: first clinical trials. J Neurosurg. 1985;62(2):169–73. https://doi.org/10.3171/jns.1985.62.2.0169.
Goetz CG, Stebbins GT 3rd, Klawans HL, Koller WC, Grossman RG, Bakay RA, et al. United Parkinson Foundation Neurotransplantation Registry on adrenal medullary transplants: presurgical, and 1- and 2-year follow-up. Neurology. 1991;41(11):1719–22.
Stoddard SL, Ahlskog JE, Kelly PJ, Tyce GM, van Heerden JA, Zinsmeister AR, et al. Decreased adrenal medullary catecholamines in adrenal transplanted parkinsonian patients compared to nephrectomy patients. Exp Neurol. 1989;104(3):218–22.
Kordower JH, Cochran E, Penn RD, Goetz CG. Putative chromaffin cell survival and enhanced host-derived TH-fiber innervation following a functional adrenal medulla autograft for Parkinson’s disease. Ann Neurol. 1991;29(4):405–12. https://doi.org/10.1002/ana.410290411.
Minguez-Castellanos A, Escamilla-Sevilla F, Hotton GR, Toledo-Aral JJ, Ortega-Moreno A, Mendez-Ferrer S, et al. Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J Neurol Neurosurg Psychiatry. 2007;78(8):825–31. https://doi.org/10.1136/jnnp.2006.106021.
Arjona V, Minguez-Castellanos A, Montoro RJ, Ortega A, Escamilla F, Toledo-Aral JJ, et al. Autotransplantation of human carotid body cell aggregates for treatment of Parkinson’s disease. Neurosurgery. 2003;53(2):321–8. https://doi.org/10.1227/01.neu.0000073315.88827.72(discussion 8–30).
Schumacher JM, Ellias SA, Palmer EP, Kott HS, Dinsmore J, Dempsey PK, et al. Transplantation of embryonic porcine mesencephalic tissue in patients with PD. Neurology. 2000;54(5):1042–50. https://doi.org/10.1212/wnl.54.5.1042.
Fink JS, Schumacher JM, Ellias SL, Palmer EP, Saint-Hilaire M, Shannon K, et al. Porcine xenografts in Parkinson’s disease and Huntington’s disease patients: preliminary results. Cell Transplant. 2000;9(2):273–8. https://doi.org/10.1177/096368970000900212.
Deacon T, Schumacher J, Dinsmore J, Thomas C, Palmer P, Kott S, et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson’s disease. Nat Med. 1997;3(3):350–3. https://doi.org/10.1038/nm0397-350.
Bakay RA, Raiser CD, Stover NP, Subramanian T, Cornfeldt ML, Schweikert AW, et al. Implantation of spheramine in advanced Parkinson’s disease (PD). Front Biosci. 2004;9:592–602. https://doi.org/10.2741/1217.
Stover NP, Bakay RA, Subramanian T, Raiser CD, Cornfeldt ML, Schweikert AW, et al. Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease. Arch Neurol. 2005;62(12):1833–7. https://doi.org/10.1001/archneur.62.12.1833.
Stover NP, Watts RL. Spheramine for treatment of Parkinson’s disease. Neurotherapeutics. 2008;5(2):252–9. https://doi.org/10.1016/j.nurt.2008.02.006.
Gross RE, Watts RL, Hauser RA, Bakay RA, Reichmann H, von Kummer R, et al. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2011;10(6):509–19. https://doi.org/10.1016/S1474-4422(11)70097-7.
Farag ES, Vinters HV, Bronstein J. Pathologic findings in retinal pigment epithelial cell implantation for Parkinson disease. Neurology. 2009;73(14):1095–102. https://doi.org/10.1212/WNL.0b013e3181bbff1c.
Gonzalez R, Garitaonandia I, Semechkin A, Kern R. Derivation of neural stem cells from human parthenogenetic stem cells. Methods Mol Biol. 2019;1919:43–57. https://doi.org/10.1007/978-1-4939-9007-8_4.
Gonzalez R, Garitaonandia I, Poustovoitov M, Abramihina T, McEntire C, Culp B, et al. Neural stem cells derived from human parthenogenetic stem cells engraft and promote recovery in a nonhuman primate model of Parkinson’s disease. Cell Transplant. 2016;25(11):1945–66. https://doi.org/10.3727/096368916X691682.
Gonzalez R, Garitaonandia I, Crain A, Poustovoitov M, Abramihina T, Noskov A, et al. Proof of concept studies exploring the safety and functional activity of human parthenogenetic-derived neural stem cells for the treatment of Parkinson’s disease. Cell Transplant. 2015;24(4):681–90. https://doi.org/10.3727/096368915X687769.
Gonzalez R, Garitaonandia I, Abramihina T, Wambua GK, Ostrowska A, Brock M, et al. Deriving dopaminergic neurons for clinical use: a practical approach. Sci Rep. 2013;3:1463. https://doi.org/10.1038/srep01463.
Garitaonandia I, Gonzalez R, Sherman G, Semechkin A, Evans A, Kern R. Novel approach to stem cell therapy in Parkinson’s disease. Stem Cells Dev. 2018;27(14):951–7. https://doi.org/10.1089/scd.2018.0001.
Kern R, Garitaonandia I, Gonzalez R, Sherman G, Semechkin A, Braine E, Nair G, Evans A. Results of an open label, dose escalating, phase 1 clinical trial evaluating the safety of a human neural stem cell based therapy in Parkinson’s disease. Neurology. 2019;92(15 Suppl.):P1.8-016.
Barker RA, Parmar M, Kirkeby A, Bjorklund A, Thompson L, Brundin P. Are stem cell-based therapies for Parkinson’s disease ready for the clinic in 2016? J Parkinsons Dis. 2016;6(1):57–63. https://doi.org/10.3233/JPD-160798.
Wang YK, Zhu WW, Wu MH, Wu YH, Liu ZX, Liang LM, et al. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Rep. 2018;11(1):171–82. https://doi.org/10.1016/j.stemcr.2018.05.010.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol. 2016;17(3):183–93. https://doi.org/10.1038/nrm.2016.8.
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–80. https://doi.org/10.1038/nbt.1529.
Takahashi J. Strategies for bringing stem cell-derived dopamine neurons to the clinic: the Kyoto trial. Prog Brain Res. 2017;230:213–26. https://doi.org/10.1016/bs.pbr.2016.11.004.
Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest. 2020;130(2):904–20. https://doi.org/10.1172/JCI130767.
Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020. https://doi.org/10.1038/s41583-019-0257-7.
Loring JF. Autologous induced pluripotent stem cell-derived neurons to treat Parkinson’s disease. Stem Cells Dev. 2018;27(14):958–9. https://doi.org/10.1089/scd.2018.0107.
‘Reprogrammed’ stem cells implanted into patient with Parkinson’s disease. Nature. 2018. https://www.nature.com/articles/d41586-018-07407-9. Accessed 4 Apr 2020.
Studer L. Strategies for bringing stem cell-derived dopamine neurons to the clinic: the NYSTEM trial. Prog Brain Res. 2017;230:191–212. https://doi.org/10.1016/bs.pbr.2017.02.008.
Kirkeby A, Parmar M, Barker RA. Strategies for bringing stem cell-derived dopamine neurons to the clinic: a European approach (STEM-PD). Prog Brain Res. 2017;230:165–90. https://doi.org/10.1016/bs.pbr.2016.11.011.
Koprich JB, Kalia LV, Brotchie JM. Animal models of alpha-synucleinopathy for Parkinson disease drug development. Nat Rev Neurosci. 2017;18(9):515–29. https://doi.org/10.1038/nrn.2017.75.
Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT). Mov Disord. 1992;7(1):2–13. https://doi.org/10.1002/mds.870070103.
Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord. 1999;14(4):572–84. https://doi.org/10.1002/1531-8257(199907)14:4%3c572:aid-mds1005%3e3.0.co;2-c.
Pahwa R, Isaacson SH, Torres-Russotto D, Nahab FB, Lynch PM, Kotschet KE. Role of the Personal KinetiGraph in the routine clinical assessment of Parkinson’s disease: recommendations from an expert panel. Expert Rev Neurother. 2018;18(8):669–80. https://doi.org/10.1080/14737175.2018.1503948.
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–22. https://doi.org/10.1056/NEJMoa1205158.
Mestre TA, Espay AJ, Marras C, Eckman MH, Pollak P, Lang AE. Subthalamic nucleus-deep brain stimulation for early motor complications in Parkinson’s disease: the EARLYSTIM trial: early is not always better. Mov Disord. 2014;29(14):1751–6. https://doi.org/10.1002/mds.26024.
Brundin P, Kordower JH. Neuropathology in transplants in Parkinson’s disease: implications for disease pathogenesis and the future of cell therapy. Prog Brain Res. 2012;200:221–41. https://doi.org/10.1016/B978-0-444-59575-1.00010-7.
Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol. 2017;298(Pt B):225–35. https://doi.org/10.1016/j.expneurol.2017.10.003.
Sardi SP, Simuni T. New Era in disease modification in Parkinson’s disease: review of genetically targeted therapeutics. Parkinsonism Relat Disord. 2019;59:32–8. https://doi.org/10.1016/j.parkreldis.2018.10.025.
Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release. 2015;219:61–75. https://doi.org/10.1016/j.jconrel.2015.08.059.
Bachoud-Levi AC. From open to large-scale randomized cell transplantation trials in Huntington’s disease: lessons from the multicentric intracerebral grafting in Huntington’s disease trial (MIG-HD) and previous pilot studies. Prog Brain Res. 2017;230:227–61. https://doi.org/10.1016/bs.pbr.2016.12.011.
Precious SV, Zietlow R, Dunnett SB, Kelly CM, Rosser AE. Is there a place for human fetal-derived stem cells for cell replacement therapy in Huntington’s disease? Neurochem Int. 2017;106:114–21. https://doi.org/10.1016/j.neuint.2017.01.016.
Maxan A, Mason S, Saint-Pierre M, Smith E, Ho A, Harrower T, et al. Outcome of cell suspension allografts in a patient with Huntington’s disease. Ann Neurol. 2018;84(6):950–6. https://doi.org/10.1002/ana.25354.
Glass JD, Hertzberg VS, Boulis NM, Riley J, Federici T, Polak M, et al. Transplantation of spinal cord-derived neural stem cells for ALS: analysis of phase 1 and 2 trials. Neurology. 2016;87(4):392–400. https://doi.org/10.1212/WNL.0000000000002889.
Baloh RH, Glass JD, Svendsen CN. Stem cell transplantation for amyotrophic lateral sclerosis. Curr Opin Neurol. 2018;31(5):655–61. https://doi.org/10.1097/WCO.0000000000000598.
Barker RA, Mason SL, Harrower TP, Swain RA, Ho AK, Sahakian BJ, et al. The long-term safety and efficacy of bilateral transplantation of human fetal striatal tissue in patients with mild to moderate Huntington’s disease. J Neurol Neurosurg Psychiatry. 2013;84(6):657–65. https://doi.org/10.1136/jnnp-2012-302441.
Hauser RA, Furtado S, Cimino CR, Delgado H, Eichler S, Schwartz S, et al. Bilateral human fetal striatal transplantation in Huntington’s disease. Neurology. 2002;58(5):687–95. https://doi.org/10.1212/wnl.58.5.687.
Cicchetti F, Lacroix S, Cisbani G, Vallieres N, Saint-Pierre M, St-Amour I, et al. Mutant hunting tin is present in neuronal grafts in Huntington disease patients. Ann Neurol. 2014;76(1):31–42. https://doi.org/10.1002/ana.24174.
Lindvall O, Sawle G, Widner H, Rothwell JC, Bjorklund A, Brooks D, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann Neurol. 1994;35(2):172–80. https://doi.org/10.1002/ana.410350208.
Sawle GV, Bloomfield PM, Bjorklund A, Brooks DJ, Brundin P, Leenders KL, et al. Transplantation of fetal dopamine neurons in Parkinson’s disease: PET [18F]6-L-fluorodopa studies in two patients with putaminal implants. Ann Neurol. 1992;31(2):166–73. https://doi.org/10.1002/ana.410310207.
Defer GL, Geny C, Ricolfi F, Fenelon G, Monfort JC, Remy P, et al. Long-term outcome of unilaterally transplanted parkinsonian patients. I. Clinical approach. Brain. 1996;119(Pt 1):41–50. https://doi.org/10.1093/brain/119.1.41.
Freeman TB, Olanow CW, Hauser RA, Nauert GM, Smith DA, Borlongan CV, et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson’s disease. Ann Neurol. 1995;38(3):379–88. https://doi.org/10.1002/ana.410380307.
Acknowledgements
Claire Henchcliffe acknowledges funding from the C.V. Starr Foundation and New York State Stem Cell Science (NYSTEM). Harini Sarva acknowledges funding from the Daisy and Paul Soros Clinical Scholar Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received by either author for the preparation of this manuscript.
Conflicts of interest
Dr Henchcliffe has received honoraria for consulting for the California Institute of Regenerative Medicine (CIRM) Grant Working Group and research funding as an investigator in a research consortium from New York State Stem Cell Science (NYSTEM). Unrelated to this manuscript she has the following disclosures: honorarium for lecture from the Houston Methodist Neurological Institute; honoraria for ad hoc advisory board participation from US WorldMeds, Adamas Pharmaceuticals, Mitsubishi Tanabe Pharma Inc., and Prevail Therapeutics; and funding from the Michael J. Fox Foundation, National Institute of Health, Insightec, and Prevail Therapeutics. Dr H Sarva has received funding from the Michael J Fox Foundation, and clinical trial support from Biogen, Insightec and Lundbeck Pharmaceuticals. She has received honoraria for participation in advisory boards for Merz and Amneal pharmaceuticals, and for serving as an independent video rater for Neurocrine Neurosciences.
Rights and permissions
About this article
Cite this article
Henchcliffe, C., Sarva, H. Restoring Function to Dopaminergic Neurons: Progress in the Development of Cell-Based Therapies for Parkinson’s Disease. CNS Drugs 34, 559–577 (2020). https://doi.org/10.1007/s40263-020-00727-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00727-3