Abstract
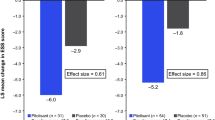
Pitolisant (Wakix®), an orally available, first-in-class antagonist/inverse agonist of the histamine 3 receptor, is approved in the EU (as of March 2016) for the treatment of narcolepsy with or without cataplexy in adults and in the USA (as of August 2019) for the treatment of excessive daytime sleepiness (EDS) in adults with narcolepsy. Pitolisant was demonstrated to have minimal risk of abuse in preclinical and clinical studies, and is the only anti-narcoleptic drug not scheduled as a controlled substance in the USA. The totality of evidence from pivotal and supportive phase III trials suggests that pitolisant administered at up to the recommended maximum dose of 36 mg once daily reduces EDS and cataplexy in adults with narcolepsy relative to placebo. Noninferiority of pitolisant to modafinil in the management of EDS was not demonstrated. Pitolisant was generally well tolerated in clinical trials. Consistent with its mechanism of action, the most common treatment-emergent adverse events included headache, insomnia and anxiety. With minimal abuse potential and offering the convenience of oral, once-daily administration, pitolisant extends the range of approved treatment options available to adult patients with narcolepsy with or without cataplexy.



Similar content being viewed by others
References
Barateau L, Lopez R, Dauvilliers Y. Management of narcolepsy. Curr Treat Options Neurol. 2016;18(43):1–13.
Ohayon MM, Priest RG, Zulley J, et al. Prevalence of narcolepsy symptomatology and diagnosis in the general European population. Neurology. 2002;58:1826–33.
de Biase S, Gigli GL, Valente M. Important decisions in choosing the pharmacotherapy for narcoleptics. Expert Opin Pharmacother. 2019;20(5):483–6.
Barateau L, Lopez R, Dauvilliers Y. Treatment options for narcolepsy. CNS Drugs. 2016;30(5):369–79.
De la Herrán-Arita AK, García-García F. Current and emerging options for the drug treatment of narcolepsy. Drugs. 2013;73(16):1771–81.
Busardò FP, Kyriakou C, Napoletano S, et al. Clinical applications of sodium oxybate (GHB): from narcolepsy to alcohol withdrawal syndrome. Eur Rev Med Pharmacol Sci. 2015;19:4654–63.
Ligneau X, Perrin D, Landais L, et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J Pharmacol Exp Ther. 2007;320(1):365–75.
Lin JS, Dauvilliers Y, Arnulf I, et al. An inverse agonist of the histamine H3 receptor improves wakefulness in narcolepsy: studies in orexin−/− mice and patients. Neurobiol Dis. 2008;30(1):74–83.
Bioprojet Pharma. Wakix (pitolisant): summary of product characteristics. 2019. http://www.ema.europa.eu/. Accessed 8 Jan 2020.
Harmony Biosciences. WAKIX® (pitolisant) tablets, for oral use: US prescribing information. 2019. http://www.accessdata.fda.gov/. Accessed 8 Jan 2020.
Riddy DM, Cook AE, Shackleford DM, et al. Drug-receptor kinetics and sigma-1 receptor affinity differentiate clinically evaluated histamine H3 receptor antagonists. Neuropharmacology. 2019;144:244–55.
Committee for Medicinal Products for Human Use. Wakix (pitolisant) public assessment report. 2015. http://www.ema.europa.eu/. Accessed 8 Jan 2020.
Dauvilliers Y, Arnulf I, Szakacs Z, et al. Long-term use of pitolisant to treat narcolepsy: HARMONY III study. Sleep. 2019;42(11):1–11.
Ligneau X, Shah RR, Berrebi-Bertrand I, et al. Nonclinical cardiovascular safety of pitolisant: comparing international conference on harmonization S7B and comprehensive in vitro pro-arrhythmia assay initiative studies. Br J Pharmacol. 2017;174(23):4449–63.
Kollb-Sielecka M, Demolis P, Emmerich J, et al. The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017;33:125–9.
Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163(4):713–21.
Uguen M, Perrin D, Belliard S, et al. Preclinical evaluation of the abuse potential of pitolisant, a histamine H3 receptor inverse agonist/antagonist compared with modafinil. Br J Pharmacol. 2013;169(3):632–44.
Huyts B, Brabant C, Tirelli E. Pitolisant and intravenous cocaine self-administration in mice. Eur J Pharmacol. 2019;851:63–8.
Dayno JM, Scart-Grès C, Robert P, et al. Evaluation of abuse potential of the narcolepsy medication pitolisant [abstract no. 0612]. Sleep. 2019;42(Suppl 1):A243–4.
Scart-Grès C, Setnik B, Lecomte JM, et al. Randomized, single dose, double-blind, 4-way crossover study determining the abuse potential of pitolisant compared to phentermine and placebo, in healthy, non-dependent recreational stimulant users [abstract no. P282 and poster]. J Sleep Res. 2018;27(Suppl 1):219–20.
Dauvilliers Y, Bassetti C, Lammers GJ, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068–75.
Szakacs Z, Dauvilliers Y, Mikhaylov V, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200–7.
Scart-Grès C, Momah C, Roy M, et al. Safety and tolerability of pitolisant in the treatment of adults with narcolepsy: integrated data from clinical studies [abstract no. 0614]. Sleep. 2019;42(Suppl 1):A244–5.
Jennum P, Ibsen R, Petersen ER, et al. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med. 2012;13(8):1086–93.
Daniels E, King MA, Smith IE, et al. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10:75–81.
Kallweit U, Bassetti C. Pharmacological management of narcolepsy with and without cataplexy. Expert Opin Pharmacother. 2017;18(8):809–17.
Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015;16:9–18.
National Institute for Health and Care Excellence. Narcolepsy with or without cataplexy in adults: pitolisant. 2017. http://www.nice.org.uk/. Accessed 8 Jan 2020.
Billiard M, Dauvilliers Y, Dolenc-Grošelj L, et al. Management of narcolepsy in adults. In: Gilhus NE, Barnes MP, Brainin M, editors. European handbook of neurological management. p. 513–28.
Franceschini C, Pizza F, Antelmi E, et al. Narcolepsy treatment: pharmacological and behavioral strategies in adults and children. Sleep Breath. 2019. https://doi.org/10.1007/s11325-019-01894-4.
Jazz Pharmaceuticals Inc. XYREM® (sodium oxybate) oral solution, CIII: US prescribing information. 2018. http://www.accessdata.fda.gov/. Accessed 8 Jan 2020.
Teva Pharmaceuticals USA Inc. PROVIGIL® (modafinil) tablets, for oral use, C-IV: US prescribing information. 2015. http://www.accessdata.fda.gov/. Accessed 8 Jan 2020.
van der Heide A, van Schie MKM, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the Sustained Attention to Response Task, Epworth Sleepiness Scale and Maintenance of Wakefulness Test. Sleep. 2015;38(7):1051–8.
Lehert P. Epworth sleepiness scale ESS: determination of a minimum clinically relevant difference [abstract]. Sleep Med. 2017;40(Suppl 1):e186–7.
Lehert P, Falissard B. Multiple treatment comparison in narcolepsy: a network meta-analysis. Sleep. 2018;41(12):1–13.
Plazzi G, Kallweit U, Caussé C, et al. A 5-year post-authorization safety study (PASS) relative to Wakix® (pitolisant) use and its long-term safety in narcolepsy with or without cataplexy in routine medical practice [abstract no. P198]. J Sleep Res. 2018;27(Suppl 1):180.
UCB Pharma S.A. Xyrem (sodium oxybate) 500 mg/mL oral solution: summary of product characteristics. 2015. http://www.ema.europa.eu/. Accessed 8 Jan 2020.
Acknowledgements
During the peer review process, the manufacturer of pitolisant was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Yvette Lamb is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Additional information for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.11369862.
The manuscript was reviewed by:S. de Biase, Neurology Unit, Department of Experimental and Clinical Medical Sciences, University of Udine Medical School, Udine, Italy; M. M. J. Muller, Department of Psychiatry, University of Mainz, Mainz, Germany; M. G. Şenol, Department of Neurology, Sağlik Bilimleri University, Istanbul, Turkey.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Pitolisant: A Review in Narcolepsy with or without Cataplexy. CNS Drugs 34, 207–218 (2020). https://doi.org/10.1007/s40263-020-00703-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00703-x