Abstract
Background
The cost utility of treatments of age-related macular degeneration (AMD) is commonly assessed using health state transition models defined by levels of visual acuity. However, there is evidence that another measure of visual function, contrast sensitivity, may be better associated with utility than visual acuity. This paper investigates the difference in cost effectiveness resulting from models based on visual acuity and contrast sensitivity using the example of bevacizumab (Avastin) for neovascular AMD. The implications of the choice of outcome on structural uncertainty in the model are investigated.
Method
Health state transition Markov models based on levels of visual acuity and contrast sensitivity are used to represent the costs, health utilities and outcomes of the Avastin for choroidal neovascular age-related macular degeneration (ABC) trial. Health states are associated with costs and utilities based on literature values. Treatment outcomes from the ABC trial are used to predict transitions between states in both models. Total costs and quality-adjusted life-years (QALYs) are calculated for a cohort of patients treated over a defined number of model cycles.
Results
Over a 5-year time horizon, a contrast sensitivity model predicts a statistically significant (p < 0.05) 25 % greater QALY gain than the visual acuity model based on 10,000 Monte Carlo simulations. Bevacizumab is more effective and less costly than the comparator in the contrast sensitivity model and the visual acuity model.
Conclusion
There is considerable structural uncertainty associated with the choice of outcome for modelling the cost effectiveness of AMD treatments. Bevacizumab has a higher incremental QALY gain and more favourable incremental cost-effectiveness ratio when cost effectiveness is assessed using contrast sensitivity outcomes compared with using visual acuity outcomes. Previous cost-effectiveness analyses may have underestimated the cost effectiveness of anti-vascular endothelial growth factor (anti-VEGF) therapy.
Similar content being viewed by others
A model based on contrast sensitivity outcomes results in a significantly greater quality-adjusted life-year gain than a model based on visual acuity outcomes |
The finding has implications for cost-effectiveness decisions for anti-vascular endothelial growth factor therapies, which have previously been based on visual acuity models |
1 Introduction
Age-related macular degeneration (AMD) causes the progressive and irreversible loss of central vision. Patients may find it harder to read, recognise faces or make out fine detail, which can have a severe impact on their quality of life [1]. Late-stage AMD is the third largest cause of blindness [2]. In the UK, there are currently estimated to be 513,000 cases of AMD and this number is predicted to increase to 679,000 cases by 2020 [3].
Neovascular (wet) AMD is caused by the development of new blood vessels in the macular. Treatment of neovascular AMD with anti-vascular endothelial growth factor (VEGF) therapy is current clinical practice in the UK National Health Service (NHS). Spending on the anti-VEGF ranibizumab (Lucentis®, Novartis AG, Switzerland) accounted for £129 million of the NHS prescribing budget in 2010, making it the third most costly drug [4].
Economic evaluations of treatments for AMD have concluded that the two anti-VEGF therapies used within the NHS, approved ranibizumab and off-label bevacizumab (Avastin®, Roche Holdings AG, Switzerland), are cost effective at commonly applied thresholds when compared with photodynamic therapy with verteporfin (vPDT) [5, 6]. A recent head-to-head comparison found no significant difference between the two drugs in terms of effectiveness [7].
Previous health economic models, including those used to develop the UK National Institute for Health and Care Excellence (NICE)’s guidelines on ranibizumab and pegaptanib for AMD, have relied on the association between visual acuity (VA) and health utility to construct Markov models [8]. Yet there is evidence that anti-VEGF therapy is also effective in reducing the deterioration in contrast sensitivity (CS), another measure of visual function.
A cost-effectiveness model based on CS outcomes may offer advantages over previous modelling techniques. First, no single visual function outcome captures health-related quality of life (HRQoL) in AMD and interventions may have a differential impact on each outcome. CS has an independent impact on health utility and has been shown to be more closely associated with HRQoL than VA. Bansback et al. [9] found CS remained a statistically significant predictor of utility even when VA was included in a regression model. VA measures the eye’s ability to resolve fine detail at high contrast, while CS measures the ability to perceive differences between light and dark [10].
Second, utility values for CS have been reported for binocular vision, so a model based on this outcome takes account of visual function in both eyes. Models based on VA outcomes alone have considered only visual function in the better-seeing eye, while the impact of the worse-seeing eye on health utility is uncertain [11]. In clinical practice, the eye with the disease will be treated, whether this is the better- or worse-seeing eye, therefore, taking account of vision in both eyes more closely reflects clinical practice.
There has only been one previous economic evaluation published that used CS. Bansback et al. investigated the cost effectiveness of vPDT and estimated an incremental cost effectiveness of approximately GBP 20,996 per quality-adjusted life-year (QALY) over 10 years compared with best supportive care [12].
From the previous model, it was not possible to compare the implications of using CS or VA on the cost effectiveness of treatments for AMD because there was no directly comparable VA model. Furthermore, in recent years, vPDT has been replaced by anti-VEGF therapy as standard clinical practice to treat AMD, so there is no estimate of the cost effectiveness of current clinical practice using CS.
The aim of this paper is to investigate how developing state transition models build around CS health states or VA health states impacts on the cost utility of treatments for AMD.
The choice of using VA or CS in the model is a case of structural uncertainty, the impact of which can only be tested by redesign of the model [13]. In this paper, two Markov models are developed based on the Avastin (bevacizumab) for choroidal neovascular age-related macular degeneration (ABC) trial, which assessed VA and CS outcomes in AMD patients (Table 1). Bevacizumab was compared with standard NHS treatment at the time of the trial, which was a mixture of vPDT, pegaptanib (Macugen®, Pfizer, USA), an alternative anti-VEGF and no treatment (sham injection) depending on the clinical diagnosis. The trial demonstrated that bevacizumab was an effective treatment in terms of both outcomes [14, 15].
2 Methods
2.1 Model Structure
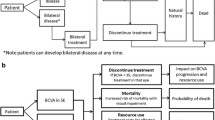
State transition Markov models were constructed to simulate the progression of the disease in terms of VA and CS. The VA model had four states of VA in the better-seeing eye and a death state. The CS had four states of binocular CS and a death state (Fig. 1). States were chosen that represented clinically relevant levels of visual function and had associated health utilities.
In the models, patients were allowed to move forwards to a better health state, move backwards to a worse health state, remain in their current health state or die at each model cycle. Death was an absorbing state, meaning that patients could not leave the state.
2.2 Transition Probabilities
Transition probabilities were calculated from patient level data on VA and CS from the ABC trial (n = 131, Table 2). Better-seeing eye VA transition rates were approximated from the study eye. The use of the better-seeing eye to assess cost effectiveness reflects that quality of life is most strongly impacted by vision in the better-seeing eye. In the trial, the study eye was the better-seeing eye for 30 % of participants. CS measurement was measured monocularly in the trial, therefore binocular CS transition rates were estimated using a published algorithm, which estimates binocular CS to be the square root of the sum of the square of each eye [16]. Age-specific mortality rates were taken from the Office for National Statistics rates for England and Wales for 2009 [17]. The rates were adjusted to take account of the sex of the cohort using the ratio of participants in the ABC trial.
The trial measured VA every 6 weeks and CS every 12 weeks for 54 weeks. The cycle length was 6 weeks for the VA model and 12 weeks for the CS model, reflecting the ABC trial protocol.
2.3 Utility
SF-6D utility values reported by Espallargues et al. [18] were applied to the health states in the model. 209 patients with unilateral or bilateral AMD at a hospital in Sheffield, UK were asked a series of preference-based questionnaires and the derived utility values were associated with their visual function. The SF-6D showed greater sensitivity than the EQ-5D, but less sensitivity than the HUI-3 to changes in vision. The SF-6D-derived utilities were chosen over the HUI-3 because the HUI-3 showed little agreement with other measures and gave extremely low utility scores compared with other measures. The HUI-3 reported a utility of just 0.10 for the worst VA state, compared with 0.63, 0.63 and 0.47 for the EQ-5D, SF-6D and time trade-off (TTO), respectively. TTO utilities were applied as sensitivity analyses. The utility values associated with levels of VA and CS were applied to the model health states (Table 3).
2.4 Cost
Resource use was estimated from the ABC trial protocol and presented in British Pounds for a cost year of 2009 (Table 4). Treatment rates were calculated from the trial to reflect that patients were not treated at every time point. If treated, costs were incurred from the drug, the examination and the consultation. Otherwise, only costs associated with the examination and consultation were incurred. A higher cost was applied to the first consultation to reflect a more extensive first visit (Table 5).
Unit costs for drugs were obtained from the British National Formulary and adjusted for the volumes used in the ABC trial. Consultation and examination costs were obtained from other published AMD models [12, 19].
2.5 Perspective
The perspective of the model was the UK NHS and personal social services (PSS) as recommended in the NICE Guide to the Methods of Technology Appraisal reference case [20]. Each model had a time horizon of 5 years, which represented an extension of the 54-week trial follow-up and captures the long-term costs and effects of the treatments. Because there is no evidence on the long-term outcomes of anti-VEGF therapy on either VA or CS, it was assumed that transition rates estimated from the 54-week trial were maintained to 5 years. A discount rate of 3.5 % for costs and QALYs was applied as recommended by the UK HM Treasury [21].
The model compared bevacizumab (1.25 mg in 0.05 mL per injection) with a comparator of mixed standard care in the UK in 2009 (16 patients received PDT, 38 patients received pegaptanib, 12 patients received sham injection) based on clinical assessment in the ABC trial.
2.6 Sensitivity Analysis
Appropriate probability functions were fitted to model parameters to incorporate uncertainty. Probabilistic sensitivity analysis was performed using a Monte Carlo simulation to randomly sample each parameter [22]. Utilities were characterised by a beta distribution, with alpha and beta parameters defined by the means and standard deviations of the utilities. Costs were characterised by a gamma distribution with alpha and beta parameters defined by the means and standard deviations of the costs. Standard deviations were not available for costs, therefore they were assumed to be 10 % of the mean in line with recommended practice for health economic models [22]. Transition probabilities were characterised by a Dirichlet distribution. A cost-effectiveness acceptability curve was constructed to represent the probability of the treatment proving cost effective at a given value of health effect [23]. One-way sensitivity analysis was employed to test structural uncertainty within the model.
3 Results
A higher incremental QALY gain is obtained from the CS model compared with the VA model. The central estimates of the probabilistic sensitivity analysis are 0.076 in the CS model and 0.061 in the VA model, which indicates that bevacizumab is 25 % more effective using CS outcomes than the VA outcomes (Table 6). This difference was statistically significant (p < 0.05) when 10,000 Monte Carlo simulations of the model were assessed using an unpaired t test.
The models indicate that bevacizumab is less costly and more effective than the comparator treatment over 5 years using either VA or CS outcomes (bevacizumab dominates the comparator).
The results remain robust when parameters were varied in sensitivity analysis. Bevacizumab dominates the comparator in all model assumptions varied in the one-way sensitivity analysis (Table 7). The CS model generates a higher incremental QALY gain than the VA model in all scenarios. The model is most sensitive to the choice of utility set.
Bevacizumab remains cost effective when a probabilistic sensitivity analysis is applied to utilities, costs and transition probabilities. Figure 2 shows the probabilistic sensitivity analysis on a cost-effectiveness plane.
The cost-effectiveness acceptability curve (CEAC) highlights that for the same cost as the comparator, bevacizumab has a probability of being cost effective of more than 60 % when assessed using VA and 65 % when assessed using CS (Fig. 3). At most values of QALY gain there is a higher probability of bevacizumab being cost effective in the CS model than in the VA model.
4 Discussion/Conclusion
The choice of outcome represents a major source of structural uncertainty when constructing models to assess the cost effectiveness of treatments for AMD and has been shown to have a large impact on cost-effectiveness estimates.
Bevacizumab appears more cost effective when assessed using CS outcomes rather than VA outcomes. In this trial, as bevacizumab dominates the comparator, the decision on the use of bevacizumab in AMD would not be altered by the choice of outcome used in the model.
The difference in incremental QALY gain between the CS and VA models when assessing the cost effectiveness of anti-VEGF therapy is potentially significant in healthcare decision making, particularly in decisions close to the cost-effectiveness threshold.
Another anti-VEGF therapy, ranibizumab, is currently recommended for the treatment of AMD patients within the NHS [8]. It has been shown to be equally effective to bevacizumab, but is more costly [7, 24]. In NICE’s economic evaluation of ranibizumab for AMD, the assessment group used a state transition model based on VA. The base-case incremental cost-effectiveness ratios (ICERs) over a 10-year time horizon for predominantly classic lesions were £15,638 per QALY gained compared with PDT, and £11,412 per QALY gained compared with best supportive care. For minimally classic lesions and occult no classic lesions, assuming 2 years of treatment, the ICER was £25,098 per QALY gained compared with best supportive care [5].
Although a direct comparison between the appraisal results and this study is not possible because of a different intervention and comparator, an improvement in cost effectiveness of 25 % could have implications on decision making at a threshold of £20,000–£30,000 per QALY, particularly in subgroups with minimally classic lesions and occult no classic lesions.
Traditionally, a CEAC such as that shown in Fig. 3 would only show positive values of health effects. However, the negative value of health effect is shown to allow inferences to be made about how the two outcomes may impact on the cost effectiveness of a more costly drug. The CEAC demonstrates that for a given value of health effect, the CS model predicts bevacizumab to be more likely to be considered cost effective.
There are two potential reasons for the different QALY estimates from the two models. First, the closer association between CS and HRQoL may mean that the CS model is more accurately representing the utility gain of the treatment than the VA model. Alternatively, the intervention may have a differential effect on VA and CS and anti-VEGF therapy may improve CS more than VA in terms of relative utility.
There are a number of limitations with this study. The comparator treatment (a mixture of pegaptanib, PDT and no treatment) as used in the ABC trial is no longer standard NHS practice because of the approval of ranibizumab. This limits interpretation of the absolute ICERs. A comparison of bevacizumab with ranibizumab based on CS outcomes would be a valuable area for future research. Furthermore, another anti-VEGF therapy, aflibercept (Eylea ®Bayer), is approved for the treatment of AMD in the US and has been shown to be equally effective compared with ranibizumab [25].
Both VA and CS have limitations when measuring very poor vision. Both measures rely on patients reading letters on a chart, so when patients cannot read the first letter, patients are assumed to have the most severe health state in the model.
Transition rates were based on trial data and allowed patients’ vision to worsen, remain the same or improve at each cycle. Anti-VEGF therapy is generally believed to maintain or reduce deterioration in vision rather than improve it. However, the nature of VA and CS as performance measures means there may be variation in the exact scores achieved by patients on each visit.
These models do not include adverse events. Of the 131 patients enrolled in the ABC trial, five patients did not complete the study because of adverse events, loss to follow-up or death. The ocular safety profiles for the two treatment groups showed no overall imbalance in serious and non-serious ocular adverse events. Given the incidence of any adverse events in the two models would be the same, their exclusion from the models should not impact on the difference between VA and CS identified.
Generally, these results highlight that the choice of clinical outcome on which a model is based can have a large impact on the cost-effectiveness estimates of the model. The uncertainty associated with the choice of clinical variable to associate with utility cannot be assigned a distribution and tested using a probabilistic sensitivity analysis, as is frequently done for costs, utilities and transition rates. Attention should be paid to the association between clinical disease states and HRQoL when developing health economic models. The clinical outcome that is best associated with HRQoL in the condition should be used where practical. If there is uncertainty over the most suitable clinical outcome for defining model states, the alternatives could be presented in a one-way sensitivity analysis.
References
Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4:97. doi:10.1186/1477-7525-4-97.
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–51 (pii S0042-96862004001100009).
Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012;96(5):752–6. doi:10.1136/bjophthalmol-2011-301109.
The prescribing year: 2011. Prescriber. 2011;22:16–7. doi:10.1002/psb.844
National Institute for Health and Care Excellence. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration. TA155. London: National Institute for Health and Care Excellence. 2008.
Hernandez-Pastor LJ, Ortega A, Garcia-Layana A, Giraldez J. Cost-effectiveness of ranibizumab compared with photodynamic treatment of neovascular age-related macular degeneration. Clin Ther. 2008;30(12):2436–51. doi:10.1016/j.clinthera.2008.12.025.
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908. doi:10.1056/NEJMoa1102673.
National Institute for Health and Care Excellence. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration. TA155. London: National Institute for Health and Care Excellence. 2012.
Bansback N, Czoski-Murray C, Carlton J, Lewis G, Hughes L, Espallargues M, et al. Determinants of health related quality of life and health state utility in patients with age related macular degeneration: the association of contrast sensitivity and visual acuity. Qual Life Res. 2007;16(3):533–43. doi:10.1007/s11136-006-9126-8.
Rubin G. Assessment of visual function in eyes with visual loss. Ophthalmol Clin N Am. 1989;2:357–67.
Brown GC, Brown MM, Sharma S, Brown HC. Patient perceptions of quality-of-life associated with bilateral visual loss. Int Ophthalmol. 1998;22(5):307–12.
Bansback N, Davis S, Brazier J. Using contrast sensitivity to estimate the cost-effectiveness of verteporfin in patients with predominantly classic age-related macular degeneration. Eye (Lond). 2007;21(12):1455–63. doi:10.1038/sj.eye.6702636.
Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force-6. Value Health J Int Soc Pharmacoecon Outcomes Res. 2012;15(6):835–42. doi:10.1016/j.jval.2012.04.014.
Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z, et al. Bevacizumab for neovascular age related macular degeneration (ABC trial): multicentre randomised double masked study. BMJ. 2010;340:c2459. doi:10.1136/bmj.c2459.
Patel PJ, Chen FK, Da Cruz L, Rubin GS, Tufail A. Contrast sensitivity outcomes in the ABC trial: a randomized trial of bevacizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):3089–93. doi:10.1167/iovs.10-6208.
Legge GE. Binocular contrast summation: II. Quadratic summation. Vis Res. 1984;24(4):385–94.
Office for National Statistics. Mortality statistics: deaths registered in 2009. 2010. Available from http://www.ons.gov.uk/ons/rel/vsob1/mortalitystatistics--deaths-registered-in-england-and-wales--series-dr-/2009/index.html. Accessed 27 Feb 2014.
Espallargues M, Czoski-Murray CJ, Bansback NJ, Carlton J, Lewis GM, Hughes LA, et al. The impact of age-related macular degeneration on health status utility values. Invest Ophthalmol Vis Sci. 2005;46(11):4016–23. doi:10.1167/iovs.05-0072.
Patel JJ, Mendes MA, Bounthavong M, Christopher ML, Boggie D, Morreale AP. Cost-utility analysis of bevacizumab versus ranibizumab in neovascular age-related macular degeneration using a Markov model. J Eval Clin Pract. 2010. doi:10.1111/j.1365-2753.2010.01546.x.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence. 2013.
The Green Book: Appraisal and Evaluation in Central Government. HM Treasury, UK. 2003.
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500.
Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–8. doi:10.1192/bjp.187.2.106.
Jackson TL, Kirkpatrick L. Cost comparison of ranibizumab and bevacizumab. BMJ. 2011;343:d5058. doi:10.1136/bmj.d5058.
Thomas M, Mousa SS, Mousa SA. Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2013;7:495–501. doi:10.2147/OPTH.S29974.
Acknowledgments
Funding: TB, PJP, AT and GSR receive funding from Moorfields Special Trustees and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Funders played no role in the design of the study, analysis of the data or preparation of the manuscript.
Conflict of interest
None.
Author Contributions
TB and GR designed the research. PP and AT collected the data and analysed the effectiveness. TB developed the economic model and wrote the manuscript. TB had final responsibility for the overall content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Butt, T., Patel, P.J., Tufail, A. et al. Modelling Cost Effectiveness in Neovascular Age-Related Macular Degeneration: The Impact of Using Contrast Sensitivity vs. Visual Acuity. Appl Health Econ Health Policy 12, 289–297 (2014). https://doi.org/10.1007/s40258-014-0090-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-014-0090-0