Abstract
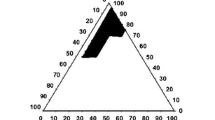
A solid self emulsifying formulation (S-SEF) has been developed with an intention to improve the dissolution characteristics of poorly water soluble lercanidipine hydrochloride (LH). Suitable components for the formulation of liquid self emulsifying drug delivery systems (SEDDS) were selected after screening various vehicles via solubility studies. Formulations were designed with Gelucire® 44/14 as oil, labrasol as surfactant and transcutol-P as co surfactant. The prepared formulations were evaluated for self emulsifying efficiency and ternary phase diagram was used to designate optimum systems in the emulsifying domain. These systems were further investigated for robustness towards different pH conditions, globule size, thermodynamic stability, surface morphology, cloud point and in vitro drug release. The optimized LH loaded formulation possessed a mean globule size of 142.5 ± 5.37 nm and cloud point of 72 ± 2.66 °C. The liquid SEDDS was transformed into free flowing S-SEF by adsorbing on to an inert carrier, Neusilin US2®. The results revealed no difference in globule size and emulsification characteristics between liquid SEDDS and S-SEF. The solid state characterization studies indicated loss of crystallinity for the drug. Significant improvement in dissolution characteristics of LH for prepared S-SEF was observed compared with pure drug.







Similar content being viewed by others
References
Aungst BJ (1993) Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J Pharm Sci 82:979–986
Bachynsky MO, Shah NH, Patel CI, Malick AW (1997) Factors affecting the efficiency of a self-emulsifying oral delivery system. Drug Dev Ind Pharm 23:809–816
Barker SA, Yap SP, Yuen KH, McCoy CP, Murphy JR, Craig DQM (2003) An investigation into the structure and bioavailability of alpha tocopherol dispersions in Gelucire 44/14. J Control Release 91:477–488
Bunjes H, Koch MHJ, Westesen K (2000) Effect of particle size on colloidal solid triglycerides. Langmuir 16:5234–5241
Carr RL (1965) Evaluating flow properties of solids. Chem Eng 18:163–168
Chambin O, Karbowiab T, Djebili L, Jannin V, Champion D, Pourcelot Y, Cayot P (2009) Influence of drug polarity upon the solid state structure and release properties of self-emulsifying drug delivery systems in relation with water affinity. Colloids Surf B 71:73–78
Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW (1992) Self emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res 9:87–93
Christensen JO, Schultz K, Mollgaard B, Kristensen HG, Mullertz A (2004) Solubilisation of poorly water soluble drugs during in vitro lipolysis of medium and long chain triacylglycerols. Eur J Pharm Sci 23:287–296
Craig DQM, Barker SA, Banning D, Booth SW (1995) An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm 114:103–110
Dixit RP, Nagarsenker MS (2008) Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci 35:183–192
Dixit G, Baldevprasad K, Pradhan NS, Valgeirsson J (2008) Lercanidipine hydrochloride polymorphs and an improved process for preparation of 1,1,N-trimethyl-N-(3,3-diphenylpropyl)-2-aminoethyl acetoacetate. US Patent No. 12/530, 101
Eccleston GM (1992) Microemulsions. In: Swarbrick S, Boylan JC (eds) Encyclopedia of pharmaceutical technology. Marcel Dekker, New York, pp 375–421
Elnaggar YSR, El-Massik MA, Abdallah OY (2009) Self-nanoemulsifying drug delivery systems of tamoxifen citrate: design and optimization. Int J Pharm 380:133–141
Fatouros DG, Deen GR, Arleth L, Bergenstahl B, Nielsen FS, Pedersen JS, Mullertz A (2007) Structural development of self-nano emulsifying drug delivery systems (SNEDDS) during in vitro lipid digestion monitored by small angle X-ray scattering. Pharm Res 24:1844–1853
Groves MJ (1976) The self-emulsifying action of mixed surfactants in oil. Acta Pharm Suec 13:361–372
Gursoy RN, Benita S (2004) Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother 58:173–182
Hauss DJ (2007) Oral lipid based formulations. Adv Drug Deliv Rev 59:667–676
Hu Z, Tawa R, Konishi T, Shibata N, Takada K (2001) A novel emulsifier, labrasol, enhances gastrointestinal absorption of gentamicin. Life Sci 69:2899–2910
Humberstone AJ, Charman WN (1997) Lipid based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev 25:103–128
Karbowiak T, Debeaufort F, Voilley A (2006) Importance of surface tension characterization for food, pharmaceutical and packaging products: a review. Crit Rev Food Sci 46:391–407
Kawakami K, Yoshikawa T, Hayashi T, Nishihara Y, Masuda K (2002) Microemulsion formulation for enhanced absorption of poorly soluble drugs: II In vivo study. J Control Release 81:75–82
Khan KA (1975) The concept of dissolution efficiency. J Pharm Pharmacol 27:48–49
Kim HJ, Yoon KA, Hahn M, Park ES, Chi SC (2000) Preparation and in vitro evaluation of self micro emulsifying drug delivery systems containing idebenone. Drug Dev Ind Pharm 26:523–529
Lawrence MJ, Rees GD (2000) Microemulsion based media as novel drug delivery systems. Adv Drug Del Rev 45:89–121
Levy MY, Benita S (1990) Drug release from submicronized o/w emulsion: a new in vitro kinetic evaluation model. Int J Pharm 66:29–37
Levy G, Miller KE, Reuning RH (1966) Effect of complex formation on drug absorption. 3. Concentration and drug dependent effect of a nonionic surfactant. J Pharm Sci 55:394–398
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26
Luo Y, Chen D, Ren L, Zhao X, Qin J (2006) Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J Control Release 114:53–59
Muller RH, Mader K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery: a review of the state of the art. Eur J Pharm Biopharm 50:161–177
Patil P, Joshi P, Paradkar A (2004) Effect of formulation variables on preparation and evaluation of gelled self-emulsifying drug delivery system (SEDDS) of ketoprofen. AAPS Pharm Sci Tech 5
Patrick JS (2006) Martin’s physical pharmacy and pharmaceutical sciences, 5th edn. Lippincott, Williams & Wilkins, Baltimore, pp 35–37
Pushp RN, Hyo-Kyung H, Hoo-Kyun C (2010) Preparation and in vitro–in vivo evaluation of Witepsol® H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q10. Eur J Pharm Sci 39:224–232
Schulman JH, Montagne JB (1961) Formation of microemulsions by amino alkyl alcohols. Ann N Y Acad Sci 92:366–371
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M (2007) Development and bioavailability assessment of ramipril nano emulsion formulation. Eur J Pharm Biopharm 66:227–243
Shahidzadeh N, Bonn D, Meunier J (2000) Dynamics of spontaneous emulsification for fabrication of oil in water emulsions. Langmuir 16:9703–9708
Sjoblom J, Stenius P, Danielession I (1987) Phase equilibrium of nonionic surfactants and the formation of microemulsion. In: Schick MJ (ed) Nonionic surfactants physical chemistry. Marcel Dekker, New York
Testa R, Leonardi A, Tajana A, Riscassi E, Magliocca R, Sartani A (1997) Lercanidipine (Rec 15/2375): a novel 1,4-dihydropyridine calcium antagonist for hypertension. Cardiovasc Drug Rev 15:187–219
Woo JS, Kim TS, Park JH, Chi SC (2007) Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch Pharm Res 30:82–89
Wu W, Wang Y, Que L (2006) Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm 63:288–294
Yuksel N, Karatas A, Ozkan Y, Savaser A, Ozkan SA, Baykara T (2003) Enhanced bioavailability of piroxicam using Gelucire 44/14 and labrasol: in vitro and in vivo evaluation. Eur J Pharm Biopharm 56:453–459
Zhang P, Liu Y, Feng N, Xu J (2008) Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int J Pharm 355:269–276
Acknowledgments
The authors are much grateful to ABITEC Corporations, Cleveland, USA, Gatteffose, France and Fuji Chemicals, Japan for providing the gift samples of excipients. The authors also thank Sun Pharma, Ahmedabad for providing gift sample of lercanidipine HCl. The financial assistance to Kallakunta Venkata Raman and Basanth Babu Eedara by All India Council of Technical Education (New Delhi, India) in the form of Junior Research Fellowship is duly acknowledged. The authors also thank Mr. T. Jayapal Reddy, Chairman, St. Peter’s Institute of Pharmaceutical Sciences, Hanamkonda for providing the necessary facilities.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kallakunta, V.R., Eedara, B.B., Jukanti, R. et al. A Gelucire 44/14 and labrasol based solid self emulsifying drug delivery system: formulation and evaluation. Journal of Pharmaceutical Investigation 43, 185–196 (2013). https://doi.org/10.1007/s40005-013-0060-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-013-0060-9