Abstract
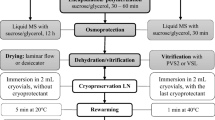
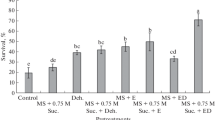
As genetic erosion of pistachio (Pistacia vera L.) has been occurring in the Mediterranean, Central and West Asia and North Africa, experiments were conducted to conserve two cultivars (‘Atlı’ and ‘Siirt’) of mature pistachio germplasm by assessing both medium- and long-term conservation techniques. In medium-term conservation, our results showed that it was feasible to conserve both cultivars in the form of either microshoots or encapsulated shoot apices up to 12 months at 4°C in the dark. As regards long-term conservation, encapsulation-dehydration and droplet-vitrification techniques were assessed for cryopreservation of cold-hardened and osmoprotected shoot apices of mature ‘Atlı’ cultivar. Among the methods tested, 13.6% of regrowth was achieved with incubation of explants in the droplets of vitrification solution for 150 min at 0°C followed by direct immersion in liquid nitrogen (LN), rapidly thawed and then cultured on Murashige and Skoog’s (MS) medium containing 1 mg L−1 BA and 0.5 mg L−1 GA3. The developed droplet-vitrification technique appeared as a promising procedure for long-term preservation of shoot apices of mature pistachio germplasm. Moreover, assesment of genetic fidelity by Random Amplified Polymorphic DNA analysis (RAPD) revealed out high levels of genetic stability between donor plant and cryopreserved plants (similarity indexes between 0.959 and 0.973) after they were subcultured for at least 3 months. The detected low level of genetic instability could be due to the toxic effect of PVS2 and regeneration phase. The optimized conservation techniques, especially slow growth storage, could be applied to preserve other Pistacia species.





Similar content being viewed by others
Abbreviations
- MC:
-
Moisture content
- MS:
-
Murashige and Skoog medium
- LN:
-
Liquid nitrogen
- PVS2:
-
Plant vitrification solution 2
- RAPD:
-
Random Amplified Polymorphic DNA
- SFC:
-
Shoot forming capacity index
References
Ashmore SE (1997) Status report on the development and application of in vitro techniques for the conservation and use of plant genetic resources. International Plant Genetic Resources Institute, Rome
Bajaj YPS (1995) Cryopreservation of plant cell, tissue, and organ culture for the conservation of germplasm and biodiversity. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 32. Springer-Verlag, Berlin Heidelberg, pp 3–28
Barazani O, Atayev A, Yakubov B, Kostiukovsky V, Popov K, Golan-Goldhirsh A (2003) Genetic variability in Turkmen populations of Pistacia vera L. Genet Resour Crop Evol 50:383–389
Barghchi M (1986) In vitro storage of plant genetic resources. Plant Physiology Division Biennial Report, Palmerston North, Department of Scientific and Industrial Research, p 52
Castillo NRF, Bassil NV, Wada S, Reed BM (2010) Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell Dev Biol-Plant 46:246–256
Chang Y, Barker RE, Reed BM (2000) Cold acclimation improves recovery of cryopreserved grass (Zoysia and Lolium sp.). CryoLetters 21:107–116
De Carlo A, Benelli C, Lambardi M (2000) Development of a shoot-tip vitrification protocol and comparison with encapsulation-based procedures for plum cryopreservation. CryoLetters 21:215–222
Dumet D, Block W, Worland R, Reed B, Benson E (2000) Profiling cryopreservation protocols for Ribes ciliatum using differential scanning calorimetry. CryoLetters 21:367–378
Engelmann F, Dussert S (2000) Current development and use of cryopreservation for the conservation of plant genetic resources. Cahiers Agricultures 9:237–245
Gagliardi R, Pacheco G, Carneiro L, Valls J, Vieira M, Mansur E (2003) Cryopreservation of Arachis species by vitrification of in vitro grown shoot apices and genetic stability of recovered plants. CryoLetters 24:103–110
Gamborg OL, Constanbel F, Shyluk JP (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Ghorbel BR, Navarro L, Duran-Vila N (1998) Morphogenesis and regeneration of whole plants of grapefruit (Citrus paradisi), sour orange (C. aurantium) and alemow (C. macrophylla). J Hort Sci Biotech 73:323–327
Gollagunta V, Adelberg JW, Rieck J, Rajapakse N (2005) Sucrose in storage media and cultivar affects post-storage regrowth of in vitro Hosta propagules. Plant Cell Tiss Org Cult 80:191–199
Hao Y, You C, Deng XX (2002) Effects of cryopreservation on developmental competency, cytological and molecular stability of Citrus callus. CryoLetters 23:27–35
Hao YJ, Deng XX (2003) Genetically stable regeneration of apple plants from slow growth. Plant Cell Tiss Org Cult 72:253–260
Hassan NS (2004) Storage of in vitro banana shoot cultures at low temperature or under mineral oil layer. Int J Agric Biol 6(2):303–306
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaudoise Sci Nat 44:223–270
Kaity A, Ashmore SE, Drew RA (2008) Assessment of genetic and epigenetic changes following cryopreservation in papaya. Plant Cell Rep 27:1529–1539
Lambardi M, Sharma KK, Thorpe TA (1993) Optimization of in vitro bud induction and plantlet formation from mature embryos of Aleppo pine (Pinus halepensis Mill.). In Vitro Cell Dev Biol 29:189–199
Lodhi MA, Guang-ning Y, Weeden NFN, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Molecular Biology Reports 6–13
Marascuilo LA, McSweeney M (1977) Post-hoc multiple comparisons in sample preparations for test of homogenesity. In: McSweeney M, Marascuilo LA (eds) Non–parametric and distribution free methods the social sciences. Books/Cole Publication, Belmont, pp 141–147
Murashige T, Skoog M (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physio Plant 15:473–497
Negash A, Krens F, Schaart J, Visser B (2001) In vitro conservation of enset slow-growth conditions. Plant Cell Tiss Org Cult 66:107–111
Nyende AB, Schittenhelm S, Wagner GM, Greef JM (2003) Production, storability, and regeneration of shoot tips of potato (Solanum tuberosum L.) encapsulated in calcium alginate hollow beads. In vitro Cell Dev Biol– Plant 39:540–544
Onay A (2000) Micropropagation of Pistachio from mature trees. Plant Cell Tiss Org Cult 60:159–162
Onay A, Jeffree CE, Yeoman MM (1996) Plant regeneration from encapsulated embryoids and an embryogenic mass of pistachio, Pistacia vera L. Plant Cell Rep 15(9):723–726
Ozden Tokatli Y, Akdemir H, Tilkat E, Onay A (2010) Current status and conservation of Pistacia germplasm. Biotechnol Adv 28:130–141
Ozden Tokatli Y, Ozudogru EA, Akdemir H, De Carlo A, Gumusel F (2009) Application of cryopreservation technology for pistachio germplasm conservation. Acta Hortic 839:245–251
Ozden Tokatli Y, Ozudogru EA, Gumusel F, Lambardi M (2007) Cryopreservation of Pistacia spp. seeds by dehydration and one-step freezing. CryoLetters 28(2):83–94
Padulosi S, Hadj-Hassan A (2001) Towards a Comprehensive Documentation and Use of Pistacia Genetic Diversity in Central & W. Asia, N. Africa & Europe, IPGRI, Rome
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Peredo EL, Arroyo-García R, Reed BM, Revilla MÁ (2008) Genetic and epigenetic stability of cryopreserved and cold-stored hops (Humulus lupulus L.). Cryobiology 57:234–241
Sakai A, Kobayashi S, Oiyama Y (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sant R, Panis B, Taylor M, Tyagi A (2008) Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia esculenta var. esculenta) accessions. Plant Cell Tiss Org Cult 92(1):107–111
Sarasan V, Cripps R, Ramsay MM, Atherton C, Mcmichen M, Prendergast G (2006) Conservation in vitro of threatened plants progress in the past decade. In vitro Cell Dev Biol– Plant 42:206–214
Singh AK, Varshney R, Sharma M, Agarwal SS, Bansal KC (2006) Regeneration of plants from alginate-encapsulated shoot tips of Withania somnifera (L.) Dunal, a medicinally important plant species. J Plant Physiol 163:220–223
Tilkat E, Onay A, Yildirim H, Ozen HC (2008) Micropropagation of mature male pistachio Pistacia vera L. J Hort Sci Biotech 83(3):328–333
Towill LE, Bonnart R (2003) Cracking in a vitrification solution during cooling or warming does not affect growth of cryopreserved mint shoot tips. CryoLetters 24:341–346
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Acknowledgements
This study was funded by a grant (209T030), from TUBITAK, the Scientific and Technological Research Council of Turkey. The study was also partially funded by a grant (2009-A10) from Gebze Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akdemir, H., Süzerer, V., Tilkat, E. et al. In vitro conservation and cryopreservation of mature pistachio (Pistacia vera L.) germplasm. J. Plant Biochem. Biotechnol. 22, 43–51 (2013). https://doi.org/10.1007/s13562-012-0109-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-012-0109-2