Abstract
Background
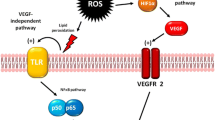
In eukaryotic cells, the cytoskeleton contains three major filamentous components: actin microfilaments, microtubules and intermediate filaments. Nestin represents one of the class VI intermediate filament proteins. Clinical and molecular analyses have revealed substantial information regarding the presence of Nestin in cells with progenitor or stem cell properties. During tissue injury Nestin is expressed in cells with progenitor cell-like properties. These cells may serve as a tissue reserve and, as such, may contribute to tissue repair. Based on currently available data, Nestin also appears to be implicated in two oncogenic processes. First, Nestin has been found to be expressed in cancer stem-like cells and poorly differentiated cancer cells and, as such, Nestin is thought to contribute to the aggressive behavior of these cells. Second, Nestin has been found to be involved in tumor angiogenesis through an interaction of cancer cells and blood vessel endothelial cells and, as such, Nestin is thought to facilitate tumor growth.
Conclusions
We conclude that Nestin may serve as a promising tumor marker and as a potential therapeutic target amenable to tumor suppression and angiogenesis inhibition.


Similar content being viewed by others
References
L. Chang, R.D. Goldman, Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5, 601–13 (2004)
U. Lendahl, L.B. Zimmerman, R.D. McKay, CNS stem cells express a new class of intermediate filament protein. Cell 60, 585–95 (1990)
M.J. Marvin, J. Dahlstrand, U. Lendahl, R.D. McKay, A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J. Cell Sci. 111, 1951–61 (1998)
G. Sjoberg, L. Edström, U. Lendahl, T. Sejersen, Myofibers from Duchenne/Becker muscular dystrophy and myositis express the intermediate filament nestin. J. Neuropathol. Exp. Neurol. 53, 416–23 (1994)
P.M. Steinert, Y.H. Chou, V. Prahlad, D.A. Parry, L.N. Marekov, K.C. Wu, S.I. Jang, R.D. Goldman, A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J. Biol. Chem. 274, 9881–90 (1990)
J. Dahlstrand, L.B. Zimmerman, R.D. McKay, U. Lendahl, Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J. Cell Sci. 103, 589–97 (1992)
Z.G. Jin, L. Liu, H. Zhong, K.J. Zhang, Y.F. Chen, W. Bian, L.P. Cheng, N.H. Jing, Second intron of mouse nestin gene directs its expression in pluripotent embryonic carcinoma cells through POU factor binding site. Acta Biochim Biophys Sin (Shanghai). 38(207–12) (2006)
L. Zimmerman, B. Parr, U. Lendahl, M. Cunningham, R. McKay, B. Gavin, J. Mann, G. Vassileva, A. McMahon, Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 12, 11–24 (1994)
R. Josephson, T. Müller, J. Pickel, S. Okabe, K. Reynolds, P.A. Turner, A. Zimmer, R.D. McKay, POU transcription factors control expression of CNS stem cell-specific genes. Development 125, 3087–100 (1998)
C. Lothian, U. Lendahl, An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur. J. Neurosci. 9, 452–62 (1997)
P.J. Yaworsky, C. Kappen, Heterogeneity of neural progenitor cells revealed by enhancers in the nestin gene. Dev. Biol. 205, 309–21 (1999)
S. Tanaka, Y. Kamachi, A. Tanouchi, H. Hamada, N. Jing, H. Kondoh, Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol. Cell. Biol. 24(8834–46) (2004)
D. Park, A.P. Xiang, F.F. Mao, L. Zhang, C.G. Di, X.M. Liu, Y. Shao, B.F. Ma, J.H. Lee, K.S. Ha, N. Walton, B.T. Lahn, Nestin is required for the proper self-renewal of neural stem cells. Stem Cells 28(2162–71) (2010)
S. Wislet-Gendebien, F. Wautier, P. Leprince, B. Rogister, Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells 23, 392–402 (2005)
Y.J. Shin, H.L. Kim, J.M. Park, J.M. Cho, S.Y. Kim, M.Y. Lee, Characterization of nestin expression and vessel association in the ischemic core following focal cerebral ischemia in rats. Cell Tissue Res. 351, 383–95 (2013)
N. Sanai, A. Alvarez-Buylla, M.S. Berger, Neural stem cells and the origin of gliomas. N. Engl. J. Med. 353, 811–22 (2005)
T. Reya, S.J. Morrison, M.F. Clarke, I.L. Weissman, Stem cells, cancer, and cancer stem cells. Nature 414, 105–11 (2001)
O. Brustle, R.D. McKay, The neuroepithelial stem cell concept: implications for neuro-oncology. J Neurooncol. 24, 57–9 (1995)
S. Bao, Q. Wu, Z. Li, S. Sathornsumetee, H. Wang, R.E. McLendon, A.B... Hjelmeland, J.N. Rich, Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 68(6043–8) (2008)
M. Dean, T. Fojo, S. Bates, Tumour stem cells and drug resistance. Nat. Rev. Cancer 5, 275–84 (2005)
P. Chinnaiyan, M. Wang, A.M. Rojiani, P.J. Tofilon, A. Chakravarti, K.K. Ang, H.Z. Zhang, E. Hammond, W. Jr Curran, M.P. Mehta, The prognostic value of nestin expression in newly diagnosed glioblastoma: report from the Radiation Therapy Oncology Group. Radiat. Oncol. 3, 32 (2008)
R.H. Dahlrot, S.K. Hermansen, S. Hansen, B.W. Kristensen, What is the clinical value of cancer stem cell markers in gliomas? Int J Clin Exp Pathol. 6, 334–48 (2013)
M. Zhang, T. Song, L. Yang, R. Chen, L. Wu, Z. Yang, J. Fang, Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J. Exp. Clin. Cancer Res. 27, 85 (2008)
F. Wan, C. Herold-Mende, B. Campos, F.S. Centner, C. Dictus, N. Becker, F. Devens, C. Mogler, J. Felsberg, N. Grabe, G. Reifenberger, P. Lichter, A. Unterberg, J.L. Bermejo, R. Ahmadi, Association of stem cell-related markers and survival in astrocytic gliomas. Biomarkers 16, 136–43 (2011)
G. Sica, G. Lama, C. Anile, M.C. Geloso, G.L.A. Torre, P. De Bonis, G. Maira, L. Lauriola, M. Jhanwar-Uniyal, A. Mangiola, Assessment of angiogenesis by CD105 and nestin expression in peritumor tissue of glioblastoma. Int. J. Oncol. 38, 41–9 (2011)
T. Milde, T. Hielscher, H. Witt, M. Kool, S.C. Mack, H.E. Deubzer, I. Oehme, M. Lodrini, A. Benner, M.D. Taylor, A. von Deimling, A.E. Kulozik, S.M. Pfister, O. Witt, A. Korshunov, Nestin expression identifies ependymoma patients with poor outcome. Brain Pathol. 22(848–60) (2012)
T. Strojnik, G.V. Røsland, P.O. Sakariassen, R. Kavalar, T. Lah, Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg. Neurol. 68, 133–43 (2007)
N. Wagner, K.D. Wagner, H. Scholz, K.M. Kirschner, A. Schedl, Intermediate filament protein nestin is expressed in developing kidney and heart and might be regulated by the Wilms’ tumor suppressor Wt1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R779–87 (2006)
J. Chen, S. Boyle, M. Zhao, W. Su, K. Takahashi, L. Davis, M. Decaestecker, T. Takahashi, M.D. Breyer, C.M. Hao, Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J. Am. Soc. Nephrol. 17, 1283–91 (2006)
T. Sakairi, K. Hiromura, S. Yamashita, S. Takeuchi, M. Tomioka, H. Ideura, A. Maeshima, Y. Kaneko, T. Kuroiwa, M. Nangaku, T. Takeuchi, Y. Nojima, Nestin expression in the kidney with an obstructed ureter. Kidney Int. 72, 307–18 (2007)
W. Liu, Y. Zhang, S. Liu, Q. Liu, J. Hao, Y. Shi, S. Zhao, H. Duan, The expression of intermediate filament protein nestin and its association with cyclin-dependent kinase 5 in the glomeruli of rats with diabetic nephropathy. Am. J. Med. Sci. 345(470–7) (2013)
B. Bussolati, S. Bruno, C. Grange, U. Ferrando, G. Camussi, Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 22, 3696–705 (2008)
S. Gupta, C. Verfaillie, D. Chmielewski, S. Kren, K. Eidman, J. Connaire, Y. Heremans, T. Lund, M. Blackstad, Y. Jiang, A. Luttun, M.E. Rosenberg, Isolation and characterization of kidney-derived stem cells. J. Am. Soc. Nephrol. 17, 3028–40 (2006)
L. da Silva Meirelles, P.C. Chagastelles, N.B. Nardi, Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 119, 2204–13 (2006)
M.D. Plotkin, M.S. Goligorsky, Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol. 291, F902–12 (2006)
E. Hunziker, M. Stein, Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem. Biophys. Res. Commun. 271, 116–9 (2000)
H. Zulewski, E.J. Abraham, M.J. Gerlach, P.B. Daniel, W. Moritz, B. Müller, M. Vallejo, M.K. Thomas, J. Habener, Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 50, 521–33 (2001)
T. Klein, Z. Ling, H. Heimberg, O.D. Madsen, R.S. Heller, P. Serup, Nestin is expressed in vascular endothelial cells in the adult human pancreas. J Histochem Cytochem 51, 697–706 (2003)
J. Lardon, I. Rooman, L. Bouwens, Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem. Cell Biol. 117, 535–40 (2002)
H. Ueno, Y. Yamada, R. Watanabe, E. Mukai, M. Hosokawa, A. Takahashi, A. Hamasaki, H. Fujiwara, S. Toyokuni, M. Yamaguchi, J. Takeda, Y. Seino, Nestin-positive cells in adult pancreas express amylase and endocrine precursor Cells. Pancreas 31, 126–31 (2005)
L. Zhang, T.P. Hong, J. Hu, Y.N. Liu, Y.H. Wu, L.S. Li, Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J. Gastroenterol. 11(2906–11) (2005)
H. Huang, X. Tang, Phenotypic determination and characterization of nestin-positive precursors derived from human fetal pancreas. Lab Invest. 83, 539–47 (2003)
R. Gao, J. Ustinov, M.A. Pulkkinen, K. Lundin, O. Korsgren, T. Otonkoski, Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 52(2007–15) (2003)
M.K. Treutelaar, J.M. Skidmore, C.L. Dias-Leme, M. Hara, L. Zhang, D. Simeone, D.M. Martin, C.F. Burant, Nestin-lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes 52(2503–12) (2003)
R.K. Humphrey, N. Bucay, G.M. Beattie, A. Lopez, C.A. Messam, V. Cirulli, A. Hayek, Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes 52, 2519–25 (2003)
N. Bardeesy, R.A. DePinho, Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2, 897–909 (2002)
C. Gilles, M. Polette, J.M. Zahm, J.M. Tournier, L. Volders, J.M. Foidart, P. Birembaut, Vimentin contributes to human mammary epithelial cell migration. J. Cell Sci. 112, 4615–25 (1999)
Y. Matsuda, Z. Naito, K. Kawahara, N. Nakazawa, M. Korc, T. Ishiwata, Nestin is a novel target for suppressing pancreatic cancer cell migration, invasion and metastasis. Cancer Biol. Ther. 11, 512–23 (2011)
M. Kawamoto, T. Ishiwata, K. Cho, E. Uchida, M. Korc, Z. Naito, T. Tajiri, Nestin expression correlates with nerve and retroperitoneal tissue invasion in pancreatic cancer. Hum. Pathol. 40, 189–98 (2009)
N. Ikeda, M. Adachi, T. Taki, C. Huang, H. Hashida, A. Takabayashi, M. Sho, Y. Nakajima, H. Kanehiro, M. Hisanaga, H. Nakano, M. Miyake, Prognostic significance of angiogenesis in human pancreatic cancer. Br. J. Cancer 79, 1553–63 (1999)
K. Kuwahara, T. Sasaki, Y. Kuwada, M. Murakami, S. Yamasaki, K. Chayama, Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas 26, 344–9 (2003)
K. Yamahatsu, Y. Matsuda, T. Ishiwata, E. Uchida, Z. Naito, Nestin as a novel therapeutic target for pancreatic cancer via tumor angiogenesis. Int. J. Oncol. 40, 1345–57 (2012)
A.P. Meert, M. Paesmans, B. Martin, P. Delmotte, T. Berghmans, J.M. Verdebout, J.J. Lafitte, C. Mascaux, J.P. Sculier, The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br. J. Cancer 87, 694–701 (2002)
D. Yamazaki, S. Kurisu, T. Takenawa, Regulation of cancer cell motility through actin reorganization. Cancer Sci. 96, 379–86 (2005)
X. Liu, Y. Zhang, [Microtubule-associated protein 2 and nestin expressions in human embryonic and fetal gastric tissues]. Nan Fang Yi Ke Da Xue Xue Bao. 32, 1328–31 (2012)
S. Dhingra, W. Feng, R.E. Brown, Z. Zhou, T. Khoury, R. Zhang, D. Tan, Clinicopathologic significance of putative stem cell markers, CD44 and nestin, in gastric adenocarcinoma. Int J Clin Exp Pathol. 4, 733–41 (2011)
T. Ishiwata, Y. Matsuda, Z. Naito, Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J. Gastroenterol. 17, 409–18 (2011)
C. Wiese, A. Rolletschek, G. Kania, A. Navarrete-Santos, S.V. Anisimov, B. Steinfarz, K.V. Tarasov, S.A. Brugh, I. Zahanich, C. Rüschenschmidt, H. Beck, P. Blyszczuk, J. Czyz, J.F. Heubach, U. Ravens, O. Horstmann, L. St-Onge, T. Braun, O. Brüstle, K.R. Boheler, A.M. Wobus, Signals from embryonic fibroblasts induce adult intestinal epithelial cells to form nestin-positive cells with proliferation and multilineage differentiation capacity in vitro. Stem Cells 24, 2085–97 (2006)
N. Teranishi, Z. Naito, T. Ishiwata, N. Tanaka, K. Furukawa, T. Seya, S. Shinji, T. Tajiri, Identification of neovasculature using nestin in colorectal cancer. Int. J. Oncol. 30, 593–603 (2007)
S. Hirota, A. Ohashi, T. Nishida, K. Isozaki, K. Kinoshita, Y. Shinomura, Y. Kitamura, Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 125, 660–7 (2003)
M. Sarlomo-Rikala, A.J. Kovatich, A. Barusevicius, M. Miettinen, CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod. Pathol. 11, 728–34 (1998)
M. van de Rijn, M.R. Hendrickson, R.V. Rouse, CD34 expression by gastrointestinal tract stromal tumors. Hum. Pathol. 25, 766–71 (1994)
M. Miettinen, J. Lasota, Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 438, 1–12 (2001)
T. Tsujimura, C. Makiishi-Shimobayashi, J. Lundkvist, U. Lendahl, K. Nakasho, A. Sugihara, T. Iwasaki, M. Mano, N. Yamada, K. Yamashita, A. Toyosaka, N. Terada, Expression of the intermediate filament nestin in gastrointestinal stromal tumors and interstitial cells of Cajal. Am. J. Pathol. 158, 817–23 (2001)
J.M. Vanderwinden, K. Gillard, M.H. De Laet, C.A. Messam, S.N. Schiffmann, Distribution of the intermediate filament nestin in the muscularis propria of the human gastrointestinal tract. Cell Tissue Res. 309, 261–8 (2002)
J. Johnston, E.B. Helwig, Granular cell tumors of the gastrointestinal tract and perianal region: a study of 74 cases. Dig. Dis. Sci. 26, 807–16 (1981)
G.B. Rossi, M. de Bellis, P. Marone, A. De Chiara, S. Losito, A. Tempesta, Granular cell tumors of the colon: report of a case and review of the literature. J. Clin. Gastroenterol. 30, 197–9 (2000)
J.R. Parfitt, C.A. McLean, M.G. Joseph, C.J. Streutker, S. Al-Haddad, D.K. Driman, Granular cell tumours of the gastrointestinal tract: expression of nestin and clinicopathological evaluation of 11 patients. Histopathology 48, 424–30 (2006)
R. Pardal, M.F. Clarke, S.J. Morrison, Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 3, 895–902 (2003)
J. Marx, Cancer research. Mutant stem cells may seed cancer. Science. 301, 1308–10 (2003)
T. Liu, F. Xu, X. Du, D. Lai, T. Liu, Y. Zhao, Q. Huang, L. Jiang, W. Huang, W. Cheng, Z. Liu, Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol. Cell. Biochem. 340, 265–73 (2010)
M.J. Pfeiffer, F.P. Smit, J.P. Sedelaar, J.A. Schalken, Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol. Med. 17, 657–64 (2011)
P. Apostolou, M. Toloudi, M. Chatziioannou, E. Ioannou, I. Papasotiriou, Cancer stem cells stemness transcription factors expression correlates with breast cancer disease stage. Curr Stem Cell Res Ther. 7, 415–9 (2012)
M. Al-Hajj, M.S. Wicha, A. Benito-Hernandez, S.J. Morrison, M.F. Clarke, Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 100, 3983–8 (2003)
C. Liu, X. Cao, Y. Zhang, H. Xu, R. Zhang, Y. Wu, P. Lu, F. Jin, Co-expression of Oct-4 and Nestin in human breast cancers. Mol. Biol. Rep. 39, 5875–81 (2012)
C. Liu, B. Chen, J. Zhu, R. Zhang, F. Yao, F. Jin, H. Xu, P. Lu, Clinical implications for nestin protein expression in breast cancer. Cancer Sci. 101, 815–9 (2010)
S. Parry, K. Savage, C. Marchiò, J.S. Reis-Filho, Nestin is expressed in basal-like and triple negative breast cancers. J. Clin. Pathol. 61, 1045–50 (2008)
H. Li, P. Cherukuri, N. Li, V. Cowling, M. Spinella, M. Cole, A.K. Godwin, W. Wells, J. DiRenzo, Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 67, 501–10 (2007)
O. Rogelsperger, C. Ekmekcioglu, W. Jäger, M. Klimpfinger, R. Königsberg, D. Krenbek, F. Sellner, T. Thalhammer, Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens. J. Pineal Res. 46, 422–32 (2009)
D.X. Tan, L.C. Manchester, M.P. Terron, L.J. Flores, R.J. Reiter, One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42, 28–42 (2007)
F. Peyrot, C. Ducrocq, Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 45, 235–46 (2008)
P.A. Witt-Enderby, N.M. Radio, J.S. Doctor, V.L. Davis, Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 41, 297–305 (2006)
V. Srinivasan, D.W. Spence, S.R. Pandi-Perumal, I. Trakht, D.P. Cardinali, Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther. 7, 189–203 (2008)
A. Korkmaz, E.J. Sanchez-Barcelo, D.X. Tan, R.J. Reiter, Role of melatonin in the epigenetic regulation of breast cancer. Breast Cancer Res. Treat. 115, 13–27 (2009)
L. Lai, L. Yuan, Q. Chen, C. Dong, L. Mao, B. Rowan, T. Frasch, S.M. Hill, The Galphai and Galphaq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. J. Pineal Res. 45, 476–88 (2008)
J.H. Lee, H.J. Park, Y.A. Kim, D.H. Lee, J.K. Noh, C.H. Kwon, S.M. Jung, S.K. Lee, The phenotypic characteristic of liver-derived stem cells from adult human deceased donor liver. Transplant. Proc. 44, 1110–2 (2012)
W.T. Bin, L.M. Ma, Q. Xu, X.L. Shi, Embryonic hepatocyte transplantation for hepatic cirrhosis: efficacy and mechanism of action. World J. Gastroenterol. 18(4), 309–22 (2012)
S. Reister, C. Kordes, I. Sawitza, D. Häussinger, The epigenetic regulation of stem cell factors in hepatic stellate cells. Stem Cells Dev. 20, 1687–99 (2011)
J.M. Luk, J. Burchard, C. Zhang, A.M. Liu, K.F. Wong, F.H. Shek, N.P. Lee, S.T. Fan, R.T. Poon, I. Ivanovska, U. Philippar, M.A. Cleary, C.A. Buser, P.M. Shaw, C.N. Lee, D.G. Tenen, H. Dai, M. Mao, DLK1-DIO3 genomic imprinted microRNA cluster at 14q32.2 defines a stemlike subtype of hepatocellular carcinoma associated with poor survival. J. Biol. Chem. 286, 30706–13 (2011)
X.R. Yang, Y. Xu, B. Yu, J. Zhou, S.J. Qiu, G.M. Shi, B.H. Zhang, W.Z. Wu, Y.H. Shi, B. Wu, G.H. Yang, Y. Ji, J. Fan, High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 59, 953–62 (2010)
M. Aihara, K. Sugawara, S. Torii, M. Hosaka, H. Kurihara, N. Saito, T. Takeuchi, Angiogenic endothelium-specific nestin expression is enhanced by the first intron of the nestin gene. Lab Invest. 84, 1581–92 (2004)
J. Mokry, D. Cízková, S. Filip, J. Ehrmann, J. Osterreicher, Z. Kolár, D. English, Nestin expression by newly formed human blood vessels. Stem Cells Dev. 13, 658–64 (2004)
S. Suzuki, J. Namiki, S. Shibata, Y. Mastuzaki, H. Okano, The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J. Histochem. Cytochem. 58, 721–30 (2010)
K. Gravdal, O.J. Halvorsen, S.A. Haukaas, L.A. Akslen, Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res. 69, 4708–15 (2009)
Y. Matsuda, M. Hagio, T. Ishiwata, Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J. Gastroenterol. 19, 42–8 (2013)
C. Calabrese, H. Poppleton, M. Kocak, T.L. Hogg, C. Fuller, B. Hamner, E.Y. Oh, M.W. Gaber, D. Finklestein, M. Allen, A. Frank, I.T. Bayazitov, S.S. Zakharenko, A. Gajjar, A. Davidoff, R.J. Gilbertson, A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82 (2007)
D. Wen, L. Ni, L. You, L. Zhang, Y. Gu, C.M. Hao, J. Chen, Upregulation of nestin in proximal tubules may participate in cell migration during renal repair. Am J Physiol Renal Physiol. 303, F1534–44 (2012)
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tampaki, E.C., Nakopoulou, L., Tampakis, A. et al. Nestin involvement in tissue injury and cancer - a potential tumor marker?. Cell Oncol. 37, 305–315 (2014). https://doi.org/10.1007/s13402-014-0193-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-014-0193-5