Abstract
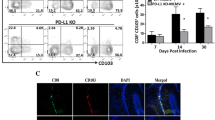
Murine cytomegalovirus (MCMV) brain infection stimulates microglial cell-driven proinflammatory chemokine production which precedes the presence of brain-infiltrating systemic immune cells. Here, we show that in response to MCMV brain infection, antigen-specific CD8(+) T cells migrated into the brain and persisted as long-lived memory cells. The role of these persistent T cells in the brain is unclear because most of our understanding of antimicrobial T cell responses comes from analyses of lymphoid tissue. Strikingly, memory T cells isolated from the brain exhibited an effector phenotype and produced IFN-γ upon restimulation with viral peptide. Furthermore, we observed time-dependent and long-term activation of resident microglia, indicated by chronic MHC class II up-regulation and TNF-α production. The immune response in this immunologically restricted site persisted in the absence of active viral replication. Lymphocyte infiltrates were detected until 30 days post-infection (p.i.), with CD8(+) and CD4(+) T cells present at a 3:1 ratio, respectively. We then investigated the role of IFN-γ in chronic microglial activation by using IFN-γ-knockout (GKO) mice. At 30 days p.i., GKO mice demonstrated a similar phenotypic brain infiltrate when compared to wild-type mice (Wt), however, MHC class II expression on microglia isolated from these GKO mice was significantly lower compared to Wt animals. When IFN-γ producing CD8(+) T cells were reconstituted in GKO mice, MHC class II up-regulation on microglial cells was restored. Taken together, these results suggest that MCMV brain infection results in long-term persistence of antigen-specific CD8(+) T cells which produce IFN-γ and drive chronic microglial cell activation. This response was found to be dependent on IFN-γ production by viral Ag-specific T cells during the chronic phase of disease.








Similar content being viewed by others
References
Bachmann MF, Wolint P, Schwarz K, Oxenius A (2005) Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol 175:4677–4685
Badovinac VP, Corbin GA, Harty JT (2000) Cutting edge: OFF cycling of TNF production by antigen-specific CD8+ T cells is antigen independent. J Immunol 165:5387–5391
Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R (2002) Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195:1541–1548
Benveniste EN (1997) Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med 75:165–173
Bergmann CC, Parra B, Hinton DR, Chandran R, Morrison M, Stohlman SA (2003) Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J Immunol 170:3204–3213
Binder GK, Griffin DE (2001) Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303–306
Blais V, Rivest S (2004) Effects of TNF-alpha and IFN-gamma on nitric oxide-induced neurotoxicity in the mouse brain. J Immunol 172:7043–7052
Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149:2736–2741
Cheeran MC, Hu S, Yager SL, Gekker G, Peterson PK, Lokensgard JR (2001) Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J Neurovirol 7:135–147
Cheeran MC, Hu S, Sheng WS, Peterson PK, Lokensgard JR (2003) CXCL10 production from cytomegalovirus-stimulated microglia is regulated by both human and viral interleukin-10. J Virol 77:4502–4515
Cheeran MC, Gekker G, Hu S, Min X, Cox D, Lokensgard JR (2004) Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. J Neurovirol 10:152–162
Cheeran MC, Gekker G, Hu S, Palmquist JM, Lokensgard JR (2005) T cell-mediated restriction of intracerebral murine cytomegalovirus infection displays dependence upon perforin but not interferon-gamma. J Neurovirol 11:274–280
Cheeran MC, Hu S, Palmquist JM, Bakken T, Gekker G, Lokensgard JR (2007) Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Res 130:96–102
Del Val M, Volkmer H, Rothbard JB, Jonjic S, Messerle M, Schickedanz J, Reddehase MJ, Koszinowski UH (1988) Molecular basis for cytolytic T-lymphocyte recognition of the murine cytomegalovirus immediate-early protein pp 89. J Virol 62:3965–3972
Farber JM (1997) Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 61:246–257
Fields BN, Knipe DM, Howley PM (2007) Fields' virology, 5th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia
Ford AL, Goodsall AL, Hickey WF, Sedgwick JD (1995) Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol 154:4309–4321
Fruh K, Yang Y (1999) Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol 11:76–81
Guidotti LG, Chisari FV (2000) Cytokine-mediated control of viral infections. Virology 273:221–227
Hamo L, Stohlman SA, Otto-Duessel M, Bergmann CC (2007) Distinct regulation of MHC molecule expression on astrocytes and microglia during viral encephalomyelitis. Glia 55:1169–1177
Harty JT, Badovinac VP (2008) Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol 8:107–119
Harty JT, Tvinnereim AR, White DW (2000) CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol 18:275–308
Hickey WF (2001) Basic principles of immunological surveillance of the normal central nervous system. Glia 36:118–124
Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL (2001) Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med 193:981–986
Holtappels R, Grzimek NK, Simon CO, Thomas D, Dreis D, Reddehase MJ (2002) Processing and presentation of murine cytomegalovirus pORFm164-derived peptide in fibroblasts in the face of all viral immunosubversive early gene functions. J Virol 76:6044–6053
Holtappels R, Simon CO, Munks MW, Thomas D, Deegen P, Kuhnapfel B, Daubner T, Emde SF, Podlech J, Grzimek NK, Oehrlein-Karpi SA, Hill AB, Reddehase MJ (2008) Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J Virol 82:5781–5796
Horner AA, Datta SK, Takabayashi K, Belyakov IM, Hayashi T, Cinman N, Nguyen MD, Van Uden JH, Berzofsky JA, Richman DD, Raz E (2001) Immunostimulatory DNA-based vaccines elicit multifaceted immune responses against HIV at systemic and mucosal sites. J Immunol 167:1584–1591
Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski UH (1990) Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol 64:5457–5464
Kosugi I, Kawasaki H, Arai Y, Tsutsui Y (2002) Innate immune responses to cytomegalovirus infection in the developing mouse brain and their evasion by virus-infected neurons. Am J Pathol 161:919–928
Koszinowski UH, Reddehase MJ, Jonjic S (1991) The role of CD4 and CD8 T cells in viral infections. Curr Opin Immunol 3:471–475
Kundig TM, Hengartner H, Zinkernagel RM (1993) T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J Immunol 150:2316–2321
Luker GD, Prior JL, Song J, Pica CM, Leib DA (2003) Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J Virol 77:11082–11093
Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR (2008) Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol 181:6417–6426
Marten NW, Stohlman SA, Zhou J, Bergmann CC (2003) Kinetics of virus-specific CD8+ − T-cell expansion and trafficking following central nervous system infection. J Virol 77:2775–2778
Masopust D, Vezys V, Marzo AL, Lefrancois L (2001) Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413–2417
Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R (2006) Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol 176:2079–2083
Matsuoka Y, Kitamura Y, Takahashi H, Tooyama I, Kimura H, Gebicke-Haerter PJ, Nomura Y, Taniguchi T (1999) Interferon-gamma plus lipopolysaccharide induction of delayed neuronal apoptosis in rat hippocampus. Neurochem Int 34:91–99
McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG (1993) Microglia in degenerative neurological disease. Glia 7:84–92
Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB (2006) Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol 176:3760–3766
Mutnal MB, Cheeran MC, Hu S, Little MR, Lokensgard JR (2010) Excess neutrophil infiltration during cytomegalovirus brain infection of interleukin-10-deficient mice. J Neuroimmunol 227:101–110
Patterson CE, Lawrence DM, Echols LA, Rall GF (2002) Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol 76:4497–4506
Perry VH, Gordon S (1988) Macrophages and microglia in the nervous system. Trends Neurosci 11:273–277
Prendergast CT, Anderton SM (2009) Immune cell entry to central nervous system–current understanding and prospective therapeutic targets. Endocr Metab Immune Disord Drug Targets 9:315–327
Quintana A, Muller M, Frausto RF, Ramos R, Getts DR, Sanz E, Hofer MJ, Krauthausen M, King NJ, Hidalgo J, Campbell IL (2009) Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J Immunol 183:2079–2088
Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH (1985) Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol 55:264–273
Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH (1987) CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol 61:3102–3108
Saito K, Yajima T, Kumabe S, Doi T, Yamada H, Sad S, Shen H, Yoshikai Y (2006) Impaired protection against Mycobacterium bovis bacillus Calmette-Guerin infection in IL-15-deficient mice. J Immunol 176:2496–2504
Slifka MK, Whitton JL (2000) Antigen-specific regulation of T cell-mediated cytokine production. Immunity 12:451–457
Slifka MK, Rodriguez F, Whitton JL (1999) Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature 401:76–79
Spencer DC, Price RW (1992) Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol 46:655–693
Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES (1994) Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol 68:6243–6253
Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ (2005) Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202:673–685
Wakim LM, Woodward-Davis A, Bevan MJ (2010) Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA 107:17872–17879
Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y (2006) IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol 176:507–515
Acknowledgments
This project was supported by Award Number R01 NS-038836 from the National Institute of Neurological Disorders and Stroke. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Presence of residual viral IE1 antigen. MCMV-infected brains were harvested at the indicated time points and were fixed and sectioned as described in the methods. Brain tissue slices (30-μm) were stained with a monoclonal antibody to IE1 followed by a fluorescein-labeled donkey anti-mouse immunoglobulin G (IgG) antibody (Jackson Immunoresearch, West Grove, PA). Immunofluorescent staining for MCMV-IE1 was performed and sections were then counter stained with 4′,6-diamidino-2-phenyindole (DAPI), a nucleic acid dye (Chemicon). Shown are the immunofluorescent staining for MCMV IE1 antigen at 5 days p.i. (a–d) and 30 days p.i., (e–h), representing different brain regions. (JPEG 10 kb)
Figure S2
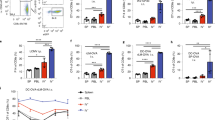
Cytokine/chemokine response in the brain. Brains from MCMV-infected Wt mice were harvested at 5 and 30 days p.i. Total RNA was extracted, DNAse treated and reverse transcribed. The cDNA obtained was analyzed by quantitative real-time PCR using primers specific to a. CXCL9 and CXCL10 b. IL7 and IL15. mRNA expression was normalized to HPRT. Mean data from three to six animals per time point are expressed as fold increase in relative mRNA induction over mock-infected controls. (JPEG 9 kb)
Figure S3
GKO mice display a similar amount of immune cell infiltration but carry excess of viral RNA in the brain. Wt and GKO mice were compared for their response to MCMV brain infection. a T cell infiltration was determined in GKO and Wt mice at 30 days p.i. Results obtained from these experiments suggested that there was no significant difference in the T cell infiltration. b MCMV-IE1 mRNA was determined in the total brain homogenates from Wt and GKO mice at 5 and 30 days p.i. by real-time PCR. At 5 days p.i., we detected significantly higher levels of IE1 expression in the brains of GKO mice when compared to Wt mice. Data are expressed as mean (±SEM) of three to five animals per group (*p < 0.05 versus MCMV-infected Wt). (JPEG 10 kb)
Rights and permissions
About this article
Cite this article
Mutnal, M.B., Hu, S., Little, M.R. et al. Memory T cells persisting in the brain following MCMV infection induce long-term microglial activation via interferon-γ. J. Neurovirol. 17, 424–437 (2011). https://doi.org/10.1007/s13365-011-0042-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-011-0042-5