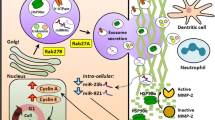
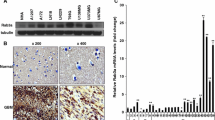
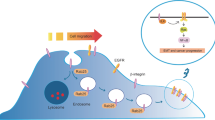
Abstract
Unlike founding members of the Ras superfamily of small GTPases that are prominently known for oncogenic signaling, members of the Rab subfamily are key regulators of cellular membrane traffic. However, a number of Rabs have in recent years also been strongly implicated as tumorigenic or metastatic biomarkers. Rab23 is an emerging example whose differential expression in tumor cells and functional association with proliferation and invasiveness is attracting attention as a useful cancer marker and a potential therapeutic target. Rab23 is ubiquitously expressed but appears to be particularly enriched in the adult brain. It has important developmental functions in vertebrates and has been shown to modulate Sonic hedgehog (Shh) and Nodal signaling. Although its exact cellular role in membrane traffic regulation remains elusive, its known role in Shh signaling, in conjunction with several recent findings, has clearly implicated a role for Rab23 in transport processes to the primary cilium. In this review, we summarize what is currently known about Rab23 as a cancer marker and discuss possible mechanism by which this Rab GTPase may act as an oncogenic or metastatic driver, while exhibiting tumor suppressive activity in some cases.

Similar content being viewed by others
References
Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25.
Kelly EE, Horgan CP, Goud B, McCaffrey MW. The Rab family of proteins: 25 years on. Biochem Soc Trans. 2012;40:1337–47.
Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196:189–201.
Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–49.
Pfeffer SR, Dirac-Svejstrup AB, Soldati T. Rab GDP dissociation inhibitor: putting Rab GTPases in the right place. J Biol Chem. 1995;270:17057–9.
Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–96.
Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol. 2004;16:451–7.
Barr F, Lambright DG, Rab GEF, GAPs. Curr Opin Cell Biol. 2010;22:461–70.
Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–63.
Horgan CP, McCaffrey MW. Rab GTPases and microtubule motors. Biochem Soc Trans. 2011;39:1202–6.
Lim YS, Tang BL. A role for Rab23 in the trafficking of Kif17 to the primary cilium. J Cell Sci. 2015;128:2996–3008.
Novick P, Medkova M, Dong G, Hutagalung A, Reinisch K, Grosshans B. Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans. 2006;34:683–6.
Chua CEL, Tang BL. Engagement of the small GTPase Rab31 protein and its effector, early endosome antigen 1, is important for trafficking of the ligand-bound epidermal growth factor receptor from the early to the late endosome. J Biol Chem. 2014;289:12375–89.
Fukuda M. TBC proteins: GAPs for mammalian small GTPase Rab? Biosci Rep. 2011;31:159–68.
Bem D, Yoshimura SI, Nunes-Bastos R, Bond FC, Bond FF, Kurian MA, Rahman F, Handley MTW, Hadzhiev Y, Masood I, Straatman-Iwanowska AA, Cullinane AR, et al. Loss-of-function mutations in RAB18 cause Warburg micro syndrome. Am J Hum Genet. 2011;88:499–507.
Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795:110–6.
Recchi C, Seabra MC. Novel functions for Rab GTPases in multiple aspects of tumour progression. Biochem Soc Trans. 2012;40:1398–403.
Chua CEL, Tang BL. The role of the small GTPase Rab31 in cancer. J Cell Mol Med. 2015;19:1–0.
Wheeler DB, Zoncu R, Root DE, Sabatini DM, Sawyers CL. Identification of an oncogenic RAB protein. Science. 2015;
Mellman I, Yarden Y. Endocytosis and cancer. Cold Spring Harb Perspect Biol. 2013;5:a016949.
Wang M, Dong Q, Wang Y. Rab23 is overexpressed in human astrocytoma and promotes cell migration and invasion through regulation of Rac1. Tumour Biol. 2016; in press.
Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–35.
Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell communication and signaling : CCS. 2010;8:23.
Bravo-Cordero JJ, Marrero-Diaz R, Megías D, Genís L, García-Grande A, García MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–510.
Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510.
Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–55.
Dozynkiewicz MA, Jamieson NB, Macpherson I, Grindlay J, van den Berghe PVE, von Thun A, Morton JP, Gourley C, Timpson P, Nixon C, McKay CJ, Carter R, et al. . Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22:131–45.
Lütcke A, Parton RG, Murphy C, Olkkonen VM, Dupree P, Valencia A, Simons K, Zerial M. Cloning and subcellular localization of novel Rab proteins reveals polarized and cell type-specific expression. J Cell Sci. 1994;107(Pt 12):3437–48.
Marcos I, Borrego S, Antiñolo G. Molecular cloning and characterization of human RAB23, a member of the group of Rab GTPases. Int J Mol Med. 2003;12:983–7.
Günther T, Struwe M, Aguzzi A, Schughart K. Open brain, a new mouse mutant with severe neural tube defects, shows altered gene expression patterns in the developing spinal cord. Development. 1994;120:3119–30.
Briscoe J, Thérond PP. The mechanisms of hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29.
Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse sonic hedgehog signalling pathway. Nature. 2001;412:194–8.
Li N, Volff JN, Wizenmann A. Rab23 GTPase is expressed asymmetrically in Hensen's node and plays a role in the dorsoventral patterning of the chick neural tube. Dev Dyn. 2007;236:2993–3006.
Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifova D, Josifiova D, Mathijssen IMJ, Morton JEV, Orstavik KH, et al. . RAB23 mutations in carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–70.
Alessandri JL, Dagoneau N, Laville JM, Baruteau J, Hébert JC, Cormier-Daire V. RAB23 mutation in a large family from Comoros Islands with carpenter syndrome. Am J Med Genet A. 2010;152A:982–6.
Jenkins D, Baynam G, De Catte L, Elcioglu N, Gabbett MT, Hudgins L, Hurst JA, Jehee FS, Oley C, Wilkie AOM. Carpenter syndrome: extended RAB23 mutation spectrum and analysis of nonsense-mediated mRNA decay. Hum Mutat. 2011;32:E2069–78.
Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev Biol. 2006;290:1–2.
Evans TM, Ferguson C, Wainwright BJ, Parton RG, Wicking C. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 2003;4:869–84.
Guo A, Wang T, Ng EL, Aulia S, Chong KH, Teng FYH, Wang Y, Tang BL. Open brain gene product Rab23: expression pattern in the adult mouse brain and functional characterization. J Neurosci Res. 2006;83:1118–27.
Huang S, Yang L, An Y, Ma X, Zhang C, Xie G, Chen ZY, Xie J, Zhang H. Expression of hedgehog signaling molecules in lung cancer. Acta Histochem. 2011;113:564–9.
Sun HJ, Liu YJ, Li N, Sun ZY, Zhao HW, Wang C, Li H, Ma FM, Shi SM, XQ X, Chen ZY, Huang SH, et al. Sublocalization of Rab23, a mediator of sonic hedgehog signaling pathway, in hepatocellular carcinoma cell lines. Mol Med Rep. 2012;6:1276–80.
Wang Y, Ng EL, Tang BL. Rab23: what exactly does it traffic? Traffic. 2006;7:746–50.
Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–7.
Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–30.
Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev. 2013;23:429–37.
Kuzhandaivel A, Schultz SW, Alkhori L, Alenius M. Cilia-mediated hedgehog signaling in Drosophila. Cell Rep. 2014;7:672–80.
Warner JF, McCarthy AM, Morris RL, McClay DR. Hedgehog signaling requires motile cilia in the sea urchin. Mol Biol Evol. 2014;31:18–22.
Kim J, Hsia EYC, Brigui A, Plessis A, Beachy PA, Zheng X. The role of ciliary trafficking in Hedgehog receptor signaling. Sci Signal. 2015;8:ra55.
Kamal R, Dahiya P, Kaur S, Bhardwaj R, Chaudhary K. Ellis-van Creveld syndrome: a rare clinical entity. Journal of oral and maxillofacial pathology : JOMFP. 2013;17:132–5.
Yang C, Chen W, Chen Y, Jiang J. Smoothened transduces Hedgehog signal by forming a complex with Evc/Evc2. Cell Res. 2012;22:1593–604.
Dorn KV, Hughes CE, Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell. 2012;23:823–35.
Barakat B, Yu L, Lo C, Vu D, De Luca E, Cain JE, Martelotto LG, Martellotto LG, Dedhar S, Sadler AJ, Wang D, Watkins DN, et al. Interaction of smoothened with integrin-linked kinase in primary cilia mediates Hedgehog signalling. EMBO Rep. 2013;14:837–44.
Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2009;106:21666–71.
Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol. 2010;191:415–28.
Cheng SY, Yue S. Role and regulation of human tumor suppressor SUFU in Hedgehog signaling. Adv Cancer Res. 2008;101:29–43.
Boehlke C, Bashkurov M, Buescher A, Krick T, John AK, Nitschke R, Walz G, Kuehn EW. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J Cell Sci. 2010;123:1460–7.
Chi S, Xie G, Liu H, Chen K, Zhang X, Li C, Xie J. Rab23 negatively regulates Gli1 transcriptional factor in a Su(Fu)-dependent manner. Cell Signal. 2012;24:1222–8.
Yoshimura SI, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–9.
Leaf A, Von Zastrow M. Dopamine receptors reveal an essential role of IFT-B, KIF17, and Rab23 in delivering specific receptors to primary cilia. eLife. 2015;4:4:e06996.
Pataki C, Matusek T, Kurucz E, Andó I, Jenny A, Mihály J. Drosophila Rab23 is involved in the regulation of the number and planar polarization of the adult cuticular hairs. Genetics. 2010;184:1051–65.
Fuller K, O'Connell JT, Gordon J, Mauti O, Eggenschwiler J. Rab23 regulates Nodal signaling in vertebrate left-right patterning independently of the Hedgehog pathway. Dev Biol. 2014;391:182–95.
Yang L, Clinton JM, Blackburn ML, Zhang Q, Zou J, Zielinska-Kwiatkowska A, Tang BL, Chansky HA. Rab23 regulates differentiation of ATDC5 chondroprogenitor cells. J Biol Chem. 2008;283:10649–57.
Huang TH, Shui HA, Ka SM, Tang BL, Chao TK, Chen JS, Lin YF, Chen A. Rab 23 is expressed in the glomerulus and plays a role in the development of focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2009;24:743–54.
Huang TH, Ka SM, Hsu YJ, Shui HA, Tang BL, KY H, Chang JL, Chen A. Rab23 plays a role in the pathophysiology of mesangial cells--a proteomic analysis. Proteomics. 2011;11:380–94.
Smith AC, Heo WD, Braun V, Jiang X, Macrae C, Casanova JE, Scidmore MA, Grinstein S, Meyer T, JH Brumell. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J Cell Biol 2007;176:263–268.
Liu YJ, Wang Q, Li W, Huang XH, Zhen MC, Huang SH, Chen LZ, Xue L, Zhang HW. Rab23 is a potential biological target for treating hepatocellular carcinoma. World J Gastroenterol. 2007;13:1010–7.
Hou Q, YH W, Grabsch H, Zhu Y, Leong SH, Ganesan K, Cross D, Tan LK, Tao J, Gopalakrishnan V, Tang BL, Kon OL, et al. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 2008;68:4623–30.
Cai ZZ, LB X, Cai JL, Wang JS, Zhou B, Hu H. Inactivation of Rab23 inhibits the invasion and motility of pancreatic duct adenocarcinoma. Genet Mol Res. 2015;14:2707–15.
Bin Z, Dedong H, Xiangjie F, Hongwei X, Qinghui Y. The microRNA-367 inhibits the invasion and metastasis of gastric cancer by directly repressing Rab23. Genetic testing and molecular biomarkers. 2015;19:69–74.
Ye F, Tang H, Liu Q, Xie X, Wu M, Liu X, Chen B, Xie X. miR-200b as a prognostic factor in breast cancer targets multiple members of RAB family. J Transl Med. 2014;12:17.
Liu Q, Tang H, Liu X, Liao Y, Li H, Zhao Z, Yuan X, Jiang W. miR-200b as a prognostic factor targets multiple members of RAB family in glioma. Med Oncol. 2014;31:859.
Song X, Sun Y, Garen A. Roles of PSF protein and VL30 RNA in reversible gene regulation. Proc Natl Acad Sci U S A. 2005;102:12189–93.
Wang G, Cui Y, Zhang G, Garen A, Song X. Regulation of proto-oncogene transcription, cell proliferation, and tumorigenesis in mice by PSF protein and a VL30 noncoding RNA. Proc Natl Acad Sci U S A. 2009;106:16794–8.
CF W, Tan GH, Ma CC, Li L. The non-coding RNA llme23 drives the malignant property of human melanoma cells. J Genet Genomics. 2013;40:179–88.
Liu Y, Zeng C, Bao N, Zhao J, Hu Y, Li C, Chi S. Effect of Rab23 on the proliferation and apoptosis in breast cancer. Oncol Rep. 2015;34:1835–44.
Kaid C, Silva PBG, Cortez BA, Rodini CO, Semedo-Kuriki P, Okamoto OK. miR-367 promotes proliferation and stem-like traits in medulloblastoma cells. Cancer Sci. 2015;106:1188–95.
Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–63.
Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol. 2011;12:222–34.
EC O, Katsanis N. Context-dependent regulation of Wnt signaling through the primary cilium. J Am Soc Nephrol. 2013;24:10–8.
Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–41.
Nachury MV. How do cilia organize signalling cascades? Philos Trans R Soc Lond Ser B Biol Sci. 2014;369.
Min TH, Kriebel M, Hou S, Pera EM. The dual regulator Sufu integrates Hedgehog and Wnt signals in the early Xenopus embryo. Dev Biol. 2011;358:262–76.
Kong JH, Yang L, Dessaud E, Chuang K, Moore DM, Rohatgi R, Briscoe J, Novitch BG. Notch activity modulates the responsiveness of neural progenitors to sonic hedgehog signaling. Dev Cell. 2015;33:373–87.
Stasiulewicz M, Gray SD, Mastromina I, Silva JC, Björklund M, Seymour PA, Booth D, Thompson C, Green RJ, Hall EA, Serup P, Dale JK, et al. A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development. 2015;142:2291–303.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26.
Gonnissen A, Isebaert S, Haustermans K. Targeting the Hedgehog signaling pathway in cancer: beyond Smoothened. Oncotarget. 2015;6:13899–913.
Alketbi A, Attoub S. Notch signaling in cancer: rationale and strategies for targeting. Curr Cancer Drug Targets. 2015;15:364–74.
Giakoustidis A, Giakoustidis D, Mudan S, Sklavos A, Williams R. Molecular signalling in hepatocellular carcinoma: role of and crosstalk among WNT/ß-catenin, Sonic Hedgehog, Notch and Dickkopf-1. Can J Gastroenterol Hepatol. 2015;29:209–17.
Christensen ST, Clement CA, Satir P, Pedersen LB. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol. 2012;226:172–84.
Farooqi AA, Waseem S, Riaz AM, Dilawar BA, Mukhtar S, Minhaj S, Waseem MS, Daniel S, Malik BA, Nawaz A, Bhatti SPDGF. The nuts and bolts of signalling toolbox. Tumour Biol. 2011;32:1057–70.
Farooqi AA, Siddik ZH. Platelet-derived growth factor (PDGF) signalling in cancer: rapidly emerging signalling landscape. Cell Biochem Funct. 2015;33:257–65.
Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, Satir P, Christensen ST, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–92.
Clement DL, Mally S, Stock C, Lethan M, Satir P, Schwab A, Pedersen SF, Christensen ST. PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J Cell Sci. 2013;126:953–65.
Umberger NL, Caspary T. Ciliary transport regulates PDGF-AA/αα signaling via elevated mammalian target of rapamycin signaling and diminished PP2A activity. Mol Biol Cell. 2015;26:350–8.
Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–71.
Hong SH, Goh SH, Lee SJ, Hwang JA, Lee J, Choi IJ, Seo H, Park JH, Suzuki H, Yamamoto E, Kim IH, Jeong JS, et al. Upregulation of adenylate cyclase 3 (ADCY3) increases the tumorigenic potential of cells by activating the CREB pathway. Oncotarget. 2013;4:1791–803.
Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol. 2014;11:714–22.
Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, KH L, Fishman D, Gray JW, Mills GB, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–6.
Cheng JM, Volk L, Janaki DKM, Vyakaranam S, Ran S, Rao KA. Tumor suppressor function of Rab25 in triple-negative breast cancer. Int J Cancer. 2010;126:2799–812.
Tang BL. Is Rab25 a tumor promoter or suppressor--context dependency on RCP status? Tumour Biol. 2010;31:359–61.
Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87.
Lim YS, Tang BL. Getting into the cilia: nature of the barrier(s). Mol Membr Biol. 2013;30:350–4.
Denning KM, Smyth PC, Cahill SF, Finn SP, Conlon E, Li J, Flavin RJ, Aherne ST, Guenther SM, Ferlinz A, O'Leary JJ, Sheils OM, et al. A molecular expression signature distinguishing follicular lesions in thyroid carcinoma using preamplification RT-PCR in archival samples. Mod Pathol. 2007;20:1095–102.
Ho JR, Chapeaublanc E, Kirkwood L, Nicolle R, Benhamou S, Lebret T, Allory Y, Southgate J, Radvanyi F, Goud B. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS One. 2012;7:e39469.
Davidson B, Abeler VM, Hellesylt E, Holth A, Shih IM, Skeie-Jensen T, Chen L, Yang Y, Wang TL. Gene expression signatures differentiate uterine endometrial stromal sarcoma from leiomyosarcoma. Gynecol Oncol. 2013;128:349–55.
Acknowledgments
BLT is supported by NUS Graduate School for Integrative Sciences and Engineering. We are grateful to the reviewers for their insightful and constructive comments, which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Chen, Y., Ng, F. & Tang, B.L. Rab23 activities and human cancer—emerging connections and mechanisms. Tumor Biol. 37, 12959–12967 (2016). https://doi.org/10.1007/s13277-016-5207-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5207-7